
Division of Cardiology, Department of Medicine, Heart Vascular Stroke Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
Copyright © 2022 Korean Society of Cardiovascular Disease Prevention; Korean Society of Cardiovascular Pharmacotherapy.
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Ethical statements
Not applicable.
Conflicts of interest
The author has no conflicts of interest to declare.
Funding
None.
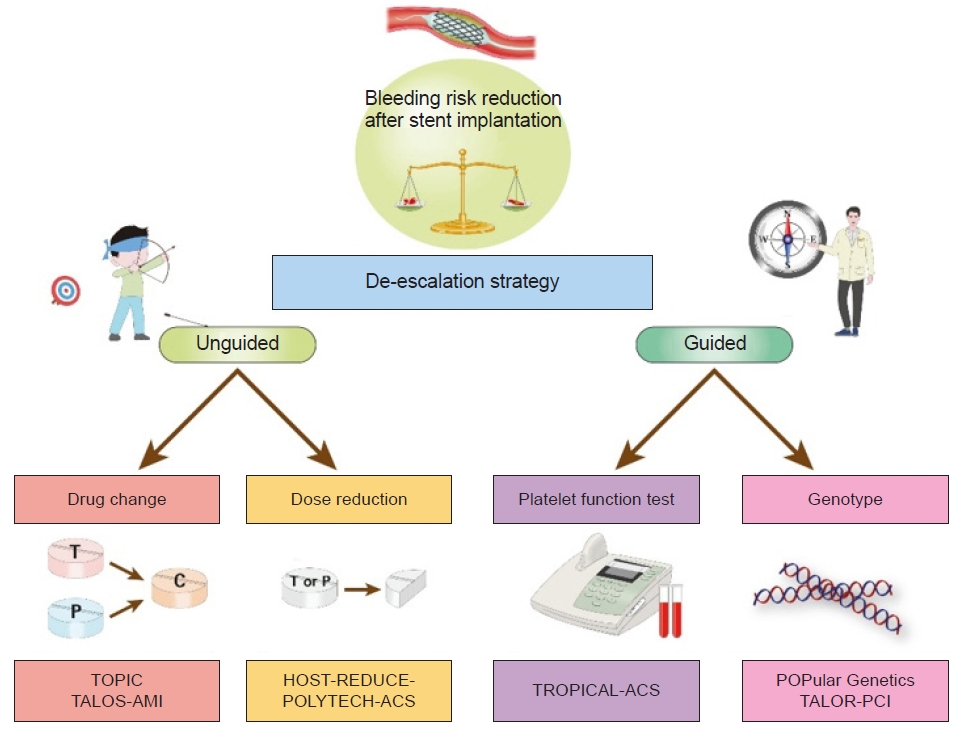
| Study | No. of patients | Design | Timing of randomization | Treatment groups | Primary endpoint | Key findings |
|---|---|---|---|---|---|---|
| TOPIC [8] | 645 | Single-center, open-label, randomized trial in ACS patients | 1 mo post-ACS | Switched DAPT (from prasugrel or ticagrelor to clopidogrel) vs. continued DAPT with prasugrel or ticagrelor | Composite of cardiovascular death, urgent revascularization, stroke, and BARC ≥2 bleeding at 1 yr | Primary endpoint: 13.4% in the switched DAPT group vs. 26.3% in the unchanged DAPT (HR, 0.48; 95% CI, 0.34–0.68; P<0.01) |
| HOST-REDUCE-POLYTECH-ACS [9] | 2,338 | Multicenter, open-label, randomized trial in ACS patients | 1 mo post-ACS | De-escalation (prasugrel 5 mg) vs. conventional (prasugrel 10 mg) | Composite of all-cause death, nonfatal MI, stent thrombosis, repeat revascularization, stroke, and BARC ≥2 bleeding at 1 yr | Primary endpoint: 7.2% in the de-escalation group vs. 10.1% in the conventional group (HR, 0.70; 95% CI, 0.52–0.92; P=0.012) |
| TALOS-AMI [10] | 2,697 | Multicenter, open-label, randomized trial in AMI patients | 1 mo post-AMI | De-escalation (from ticagrelor to clopidogrel) vs. active control (continued ticagrelor) | Composite of cardiovascular death, MI, stroke, or BARC 2, 3, or 5 bleeding at 1 yr post-AMI | Primary endpoint: 4.6% in the de-escalation group and 8.2% in the active control group (HR, 0.55; 95% CI, 0.40–0.76; P=0.0001) |
| TROPICAL-ACS [11,12] | 2,610 | Multicenter, open-label, randomized trial in biomarker-positive ACS patients with successful PCI | 2 wk post-ACS | PFT guided de-escalation to clopidogrel vs. continued prasugrel | Composite of cardiovascular death, MI, stroke, and BARC ≥2 bleeding at 1 yr | Primary endpoint: 7% in the guided de-escalation group vs. 9% in the continued prasugrel group (HR, 0.81; 95% CI, 0.62–1.06; P=0.0004 for noninferiority; P=0.12 for superiority) |
| POPular Genetics [13] | 2,488 | Multicenter, open-label, randomized trial in STEMI patients undergoing primary PCI | 1–3 day after primary PCI | Genotype-guided therapy (ticagrelor or prasugrel in LOF alleles carriers and clopidogrel in noncarriers) vs. standard treatment with ticagrelor or prasugrel | Composite of death from any cause, MI, definite stent thrombosis, stroke, or PLATO major bleeding at 1 yr | Primary endpoint: 5.1% in the genotype-guided group vs. 5.9% in the standard-treatment group (HR, 0.87; 95% CI, 0.62–1.21; P<0.001 for noninferiority; P=0.40 for superiority) |
| PLATO major or minor bleeding at 1 yr | PLATO major or minor bleeding: 9.8% in the genotype-guided group vs. 12.5% in the standard-treatment group (HR, 0.78; 95% CI, 0.61–0.98; P=0.04) |
DAPT, dual antiplatelet therapy; ACS, acute coronary syndrome; TOPIC, Timing of Platelet Inhibition after Acute Coronary Syndrome; BARC, Bleeding Academic Research Consortium; HR, hazard ratio; CI, confidence interval; HOST-REDUCE-POLYTECH-ACS, Harmonizing Optimal Strategy for Treatment of Coronary Artery Diseases-Comparison of Reduction of Prasugrel Dose or Polymer Technology in Acute Coronary Syndrome Patients; MI, myocardial infarction; TALOS-AMI, Ticagrelor versus Clopidogrel in Stabilized Patients with Acute Myocardial Infarction; AMI, acute myocardial infarction; TROPICAL-ACS, Testing Responsiveness To Platelet Inhibition On Chronic Antiplatelet Treatment For Acute Coronary Syndromes; PCI, percutaneous coronary intervention; PFT, platelet function testing; POPular Genetics, CYP2C19 Genotype-Guided Antiplatelet Therapy in ST-Segment Elevation Myocardial Infarction Patients — Patient Outcome after Primary Percutaneous Coronary Intervention; STEMI, ST-segment elevation myocardial infarction; LOF, loss of function; PLATO, PLATelet inhibition and patient Outcomes.
