Update on the Pharmacotherapy of Heart Failure with Reduced Ejection Fraction
Article information
Abstract
Heart failure (HF) is an important cardiovascular disease because of the increasing prevalence, high morbidity and mortality, and rapid expansion of health care costs. Over the past decades, efforts have been made to modify the prognosis of patients with HF. Regarding HF with reduced ejection fraction (HFrEF), several drugs have shown to improve mortality and morbidity, based on large-scale randomized controlled trials, leading to a critical paradigm shift in its pharmacological treatment. The paradigm of HFrEF pathophysiology has shifted from cardiorenal disease to hemodynamic changes, and neurohormonal activation is currently considered the prime pathophysiological mechanism of HFrEF. This review summarizes evidence on the pharmacological management of HFrEF derived from major randomized controlled trials, which have accomplished improvements in survival benefits.
INTRODUCTION
Heart failure (HF) is a clinical syndrome caused by a cardiac abnormality and characterized by typical signs and symptoms.1-3) Left ventricular ejection fraction (LVEF) has traditionally been used to classify HF. In general, patients with a LVEF less than 40% are classified as having HF with reduced ejection fraction (HFrEF), patients with LVEF within the range of 40–49% are classified as having HF with mid-range ejection fraction (HFmrEF), and patients with LVEF exceeding 50% are classified as having HF with preserved ejection fraction (HFpEF).1)
The prevalence of HF in the United States was 5.7 million in 2009, and increased to 6.2 million in 2013.4) Between 2005 and 2010, 50% of patients hospitalized with HF showed a reduced ejection fraction.4) Similarly, in Korea, the prevalence of HF increased from 0.37 million in 2002 to 0.75 million in 2013, which was 0.75% and 1.53% of total Korean population, respectively.5) As the population ages, there may be a substantial increase in the epidemiologic burden of HF with diverse comorbidities such as diabetes, hypertension, and functional mitral regurgitation.6-8)
Since the 1980s, clinical studies have made aimed to improve the survival of patients with HFrEF. A timeline of patient enrollment in clinical trials related to the pharmacological treatment of HFrEF is shown in Figure 1. In this review, we discuss drugs that have shown to improve the mortality of patients with HFrEF, and identify other attempts that have been made to improve the survival of these patients.

Timeline of patient enrollment (from the first year of enrollment) in clinical trials related to heart failure with reduced ejection fraction.
ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; ARNI = angiotensin receptor–neprilysin inhibitor; BB = beta-blocker; CCB = calcium channel blocker; DRI = direct renin inhibitor; ERA = endothelin receptor antagonist; H-ISDN = hydralazine-isosorbide dinitrate; MRA = mineralocorticoid receptor antagonist; PDE = phosphodiesterase; sGC = soluble guanylate cyclase; SGLT2 = sodium-glucose co-transporter-2.
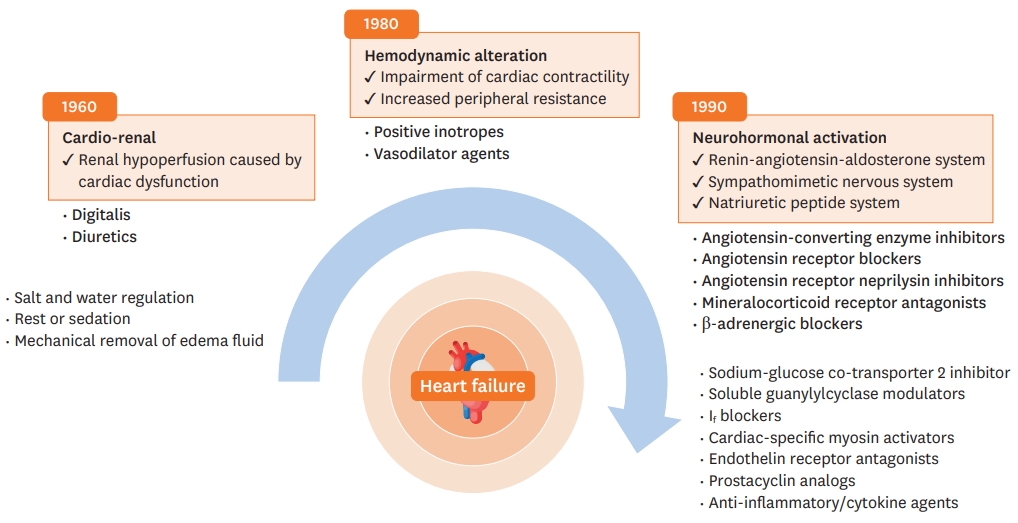
CHANGES IN HEART FAILURE TREATMENT TARGETS
With the progress in the understanding of the pathophysiology of HF, there have been many advances in treatment and diagnosis of HF, including advances in cardiac imaging, biomarkers, and genetic testing.9) The conceptual models of HF have evolved over a few decades, with several leaps in the pharmacological treatment of HF, mainly HFrEF.10)11) Figure 2 shows changes in the concept of HF pathophysiology.
In the 1940s, HF was mainly explained by cardiac dysfunction resulting in renal hypoperfusion. Early treatments for HF aimed to control symptoms, such as dyspnea and edema, and included diuretics, water and salt restriction, bed rest, and mechanical removal of edema fluid. In the 1960s, the hemodynamic model emerged and reducing left ventricular loading became a treatment target. Soon, vasodilators received attention for reducing peripheral resistance. Additionally, positive inotropes were used to increase cardiac contractility. Although these treatments alleviated patients' symptoms, they had no significant effect on the survival rate. In the 1980s, the neurohormonal model became the most prominent explanation of HFrEF pathophysiology. This model consists of three axes: the sympathetic nervous system (SNS), renin-angiotensin-aldosterone system (RAAS), and natriuretic peptide (NP) system. By targeting these systems, improvements in survival have been demonstrated in randomized controlled trials (RCTs) with angiotensin-convertingenzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), angiotensin receptorneprilysin inhibitor (ARNI), beta-blockers (BBs), and mineralocorticoid receptor antagonists (MRAs). Currently, pharmacological treatments targeting neurohormonal activation are the mainstay of various chronic heart failure (CHF) guidelines.1)
Various additional treatment strategies for HF were identified, which attempted to reinforce the neurohormonal model or to improve outcomes for patients with HF. Some of these succeeded and were incorporated into new guidelines. However, despite efforts, there remains a gap between real-life practice and guideline-directed medical treatment.12)
Current pharmacological treatment for HFrEF with survival benefits
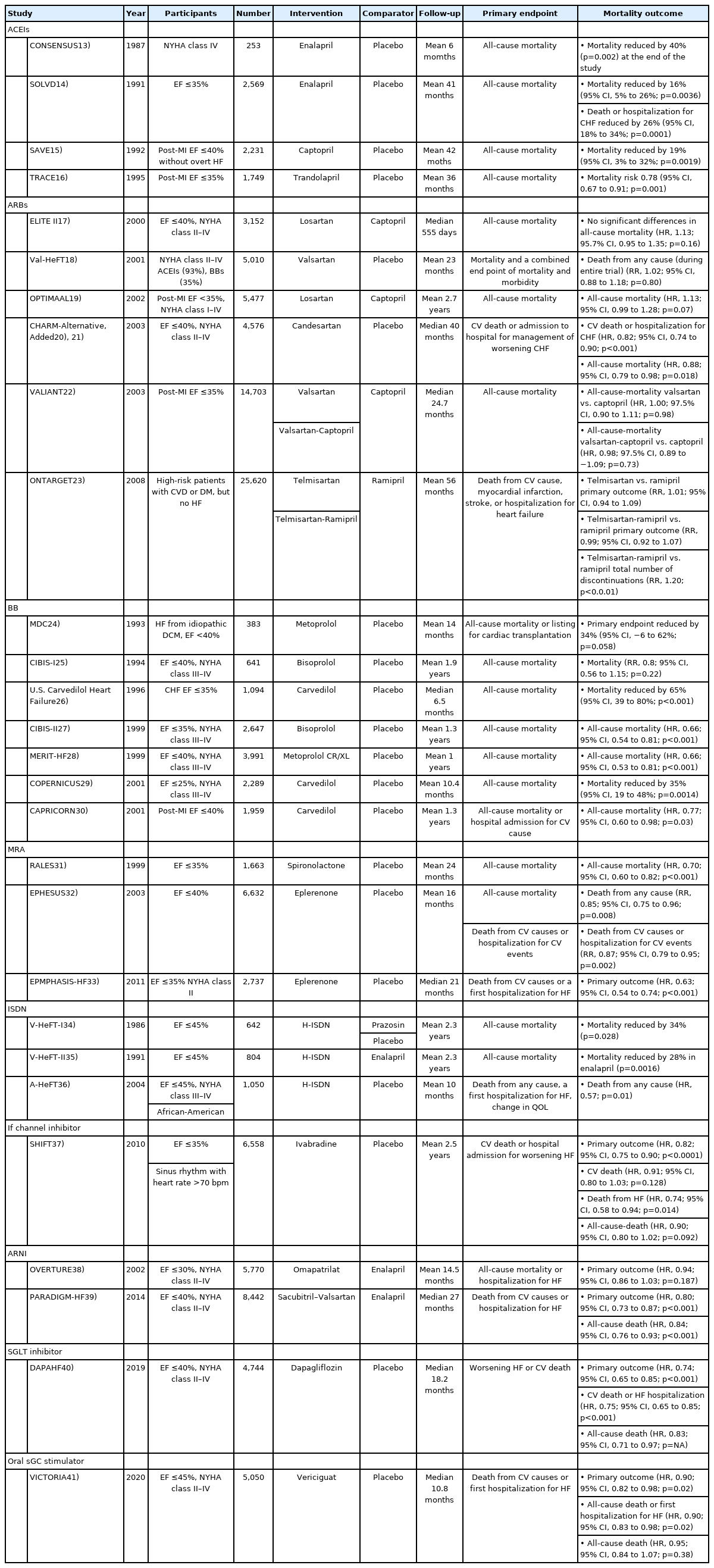
Several clinical trials have demonstrated significant survival benefits for patients with HFrEF. Table 113-41) summarizes clinical trials on drugs that reduce mortality in patients with HFrEF. Here, we review the current pharmacological treatment of HFrEF.1)42)43)
ACEIs
Currently, ACEIs are the main drugs used to treat HF. The effectiveness of ACEIs in patients with HFrEF has been validated in several clinical studies. Data from the CONSENSUS trial were published in 1988, and showed that enalapril reduced mortality by 40% over 6 months and by 27% at the end of the study compared to placebo in 253 patients with chronic heart failure (CHF).13)
Based on the CONSENSUS trial, the SOLVD trial enrolled a larger population of patients with HFrEF (n=2,569), and showed that enalapril reduced the mortality risk by 16% and hospitalization for CHF by 26% compared with placebo.14) The SAVE and TRACE trials compared the effects of captopril and trandolapril with placebo in patients with asymptomatic left ventricular (LV) dysfunction after myocardial infarction (MI). Both trials showed reduced morbidity and mortality in the ACEI group compared with the placebo group.15)16)
ARBs
ARBs were expected to provide an effective alternative treatment for patients with intolerance to ACEIs, by inhibiting RAAS through a different mechanism than ACEIs.44) In the ELITE II trial published in 2000, the effects of losartan and captopril were compared in 3,152 patients with HFrEF. There were no significant differences in total deaths, sudden deaths, or resuscitated arrests and hospital admissions. In addition, fewer patients in the losartan group discontinued medications from any adverse effect or cough.17) Losartan demonstrated non-inferiority to captopril with regards to survival in the OPTIMAAL trial.19) In the VALIANT trial, valsartan demonstrated non-inferiority compared to captopril. However, the combination of valsartan and captopril presented no survival benefit, but was associated with more frequent drug-related adverse events.22)
Combined treatment with an ACEI and an ARB has been evaluated in RCTs in patients with HF. The CHARM program,20)21) Val-HeFT,18) and later the ONTARGET trial, confirmed that the combination of ACEI and ARB resulted in a higher frequency of hypotensive symptoms, syncope, and renal dysfunction than ARB monotherapy.23) On the basis of these results, combined treatment with an ACEI and an ARB is not recommended in the current guidelines due to an increased risk of adverse events.
BBs
Early studies using metoprolol, bisoprolol, and carvedilol were conducted to determine whether beta-blockade affects the overall survival of patients with HF. The MDC trial compared metoprolol24); the CIBIS trial compared bisoprolol24); and the U.S. Carvedilol Heart Failure Study compared carvedilol with placebo in patients with HFrEF. Carvedilol therapy, as compared with placebo, resulted in a 65% reduction in overall mortality and a 27% reduction in the risk of hospitalization for cardiovascular causes.26) Later, CIBIS-II27) and MERIT-HF28) trials were performed in a larger population with bisoprolol and metoprolol CR/ XL, and significant reductions were reported in all-cause mortality compared with placebo when treated with ACEI. The survival benefits of BBs in patients with acute myocardial infarction (AMI) and LV dysfunction, and in patients with severe HF were also reported in the COPERNICUS29) and CAPRICORN30) trials.
MRAs
As the importance of the RAAS in HF pathophysiology becomes evident, the role of aldosterone is highlighted. The RALES trial showed that spironolactone, an aldosterone-receptor antagonist, can improve survival in patients with CHF.31) In patients with AMI complicated by LV dysfunction, eplerenone demonstrated survival benefits in the EPHESUS trial.32) A reduction in mortality was observed with eplerenone in EMPHASIS-HF, which enrolled patients with mild symptoms of New York Heart Association (NYHA) functional class II.33)
Hydralazine-isosorbide dinitrate (H-ISDN)
Hydralazine and nitrates act on venous capacitance and arteriolar resistance vessels, respectively, reducing preload and afterload in patients with HF.45) The V-HeFT I trial, published in 1986, compared the effects of a combination of H-ISDN, prazosin, with placebo on mortality in patients with CHF. The use of H-ISDN was found to reduce all-cause mortality. However, the trial was limited to a relatively small population of 642 patients, and only included males.34) In V-HeFT II, the effects of enalapril and H-ISDN were compared in 804 male patients with CHF. The overall mortality was higher in the H-ISDN group.35) As neurohormonal blockers became standard treatment for HF, the A-HeFT trial compared the effects of H-ISDN with placebo in patients receiving standard HF therapy. The trial included 1,050 black patients, and tested the hypothesis that vasodilator therapy might be more effective in black patients based on the results of previous trials. The use of H-ISDN resulted in a significant reduction in mortality compared with placebo.36)
If channel inhibitors
In patients with coronary artery disease and LV dysfunction, elevated heart rate (70 bpm or higher) is associated with an increased risk of cardiovascular (CV) outcomes.46) Ivabradine is a specific inhibitor of the If-current in the sinoatrial node, which slows the heart rate in patients with sinus rhythm.47) In the SHIFT trial, ivabradine was compared with placebo in patients with HF receiving conventional therapy. The trial enrolled 6,558 patients with an ejection fraction of less than 35% and a resting heart rate greater than 70 bpm, and followed them for an average of 22.9 months. At the end of the study, the heart rate of patients in the ivabradine group was 8.1 bpm lower than that of patients in the placebo group. Furthermore, the primary endpoint, a composite of CV death or hospital admission for worsening HF, was also significantly lower in the ivabradine group. All-cause mortality was lower in the ivabradine group, although the difference was not statistically significant. However, there were fewer deaths from HF, all-cause hospital admissions, and serious adverse events.37)
ARNIs
Clinical studies have also investigated the natriuretic peptide (NP) system, which represents one of the neurohormonal mechanisms of HF. Omapatrilat is a combination drug comprising an ACEI and a neprilysin inhibitor. In patients with HFrEF, omapatrilat and enalapril were compared in the OVERTURE trial, which was published in 2002. Omapatrilat was shown to reduce the risk of death and hospitalization in patients with CHF, but was not more effective than ACEI alone in reducing the risk of a primary clinical event. In terms of adverse events, angioedema was reported more frequently in the omapatrilat group compared with the enalapril group.38) Additionally, frequent angioedema was observed in another clinical trial using omapatrilat.48)
The effect of ARNI, a drug combining sacubitril and valsartan, was investigated on angioedema in the PARADIGM-HF trial, which included 8,442 patients with CHF and an ejection fraction less than 40%, and followed-up for a median duration of 27 months. ARNI and enalapril were compared for death from CV causes or hospitalization for HF as a primary outcome. This trial was terminated early due to significant primary outcome improvement in the ARNI group with a hazard ratio (HR) of 0.80. This beneficial effect was observed in death from any cause, with no major adverse events compared to enalapril.39) More recent clinical trials with ARNI will broaden the eligibility of this drug in HFrEF patients.49)
Sodium-glucose co-transporter 2 (SGLT) inhibitor
SGLT2 inhibitors represent a novel class of anti-hyperglycemic agents, which increase urinary excretion of glucose in the renal tubules.50) Trials investigating cardiovascular outcomes with empagliflozin,51) canagliflozin,52) and dapagliflozin53) demonstrated improved clinical outcomes, especially in terms of HF hospitalization. The previous clinical trials targeted diabetic patients, while the DAPA-HF trial enrolled patients with HF irrespective of diabetic status. In total, 4,744 patients with HF and an ejection fraction of ≤40% were randomly assigned to receive dapagliflozin or placebo, in addition to standard medical therapy. The primary outcome was a composite of worsening HF (hospitalization or an urgent visit resulting in intravenous therapy for HF) or cardiovascular CV death. Among patients with HFrEF, dapagliflozin reduced the primary endpoint by 26% compared with the control. In addition, the dapagliflozin group had a lower risk of all-cause mortality without an excess risk of significant adverse events, such as severe hypoglycemia.40)
Oral soluble guanylate cyclase (sGC) stimulators
As our understanding of the mechanisms underlying HF has increased, several novel signaling pathways have been identified, including the nitric oxide (NO)-sGC-cyclic guanylyl monophosphate (cGMP) pathway. Agonists of sGC can increase cGMP production, which has a protective effect on cells and tissues, including the prevention of ventricular hypertrophy and fibrosis.54) Recently, vericiguat, an oral sGC stimulator, demonstrated good clinical outcomes in patients with an ejection fraction less than 45%. During a median period of 10.8 months, there were significantly fewer primary endpoint events (death from CV causes or first hospitalization for HF) with an HR of 0.90, among patients in the vericiguat group compared with control group. Moreover, a composite of all-cause mortality or first hospitalization for HF was also lower in the vericiguat group.41)
Other drugs investigated in clinical trials of HF
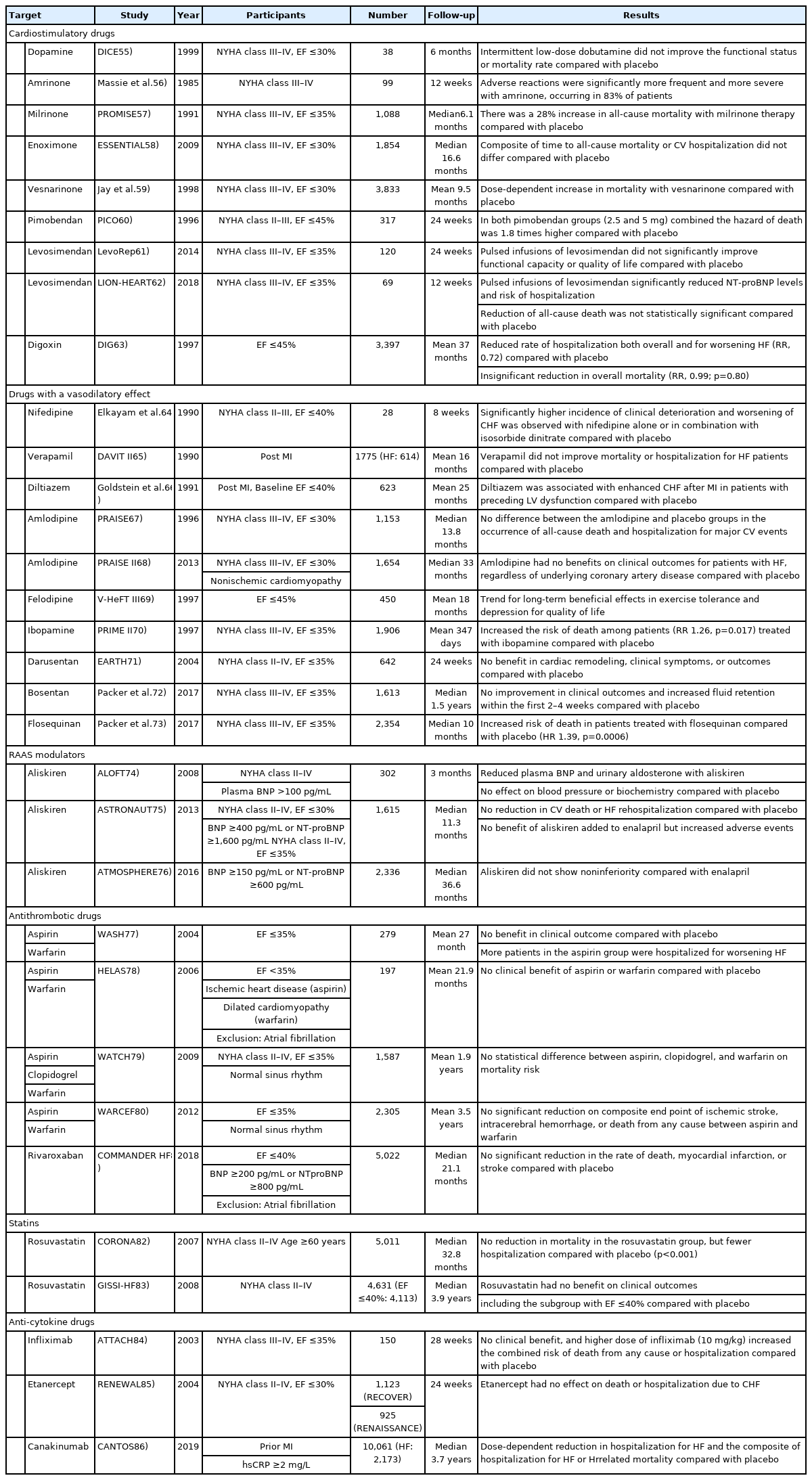
With the advent of drugs targeting the RAAS, the mortality rate of patients with HFrEF has improved significantly. However, other efforts have been made to improve the outcomes of these patients. Such efforts aimed to augment cardiac systolic function, induce vasodilation, and target other molecules associated with the RAAS. Additionally, attempts have been made to treat patients with HFrEF based on novel molecular mechanisms; not all have been successful in lowering the mortality rate. Here, we discuss studies that despite failing to reach their primary endpoint, provided meaningful insights. These studies are summarized in Table 2. 55-86)
STRENGTHENING MYOCARDIAL CONTRACTILITY
Dobutamine
Dobutamine is a sympathetic adrenergic receptor agonist that has an inotropic effect, and is useful in patients with acute refractory HF. However, it was not clear whether dobutamine would be effective in patients with severe CHF when used continuously. In the DICE trial, patients with advanced HFrEF were subjected to intermittent low-dose dobutamine infusion in addition to standard therapy for 6 months, and the outcomes were compared with placebo. Patients tolerated treatment well, but there was no improvement in mortality or functional capacity compared with placebo.55)
Phosphodiesterase inhibitors
Increased cyclic adenosine monophosphate (cAMP) in the heart induces a positive chronotropic and inotropic effect, and phosphodiesterases (PDEs) are involved in cAMP breakdown. Several subtypes of PDE exist, including PDE3, which is found in cardiac myocytes and has been studied widely.87) Amrinone is an early class drug that inhibits PDE3. However, in a trial recruiting patients with advanced HF, the use of amrinone increased the incidence of adverse effects without any significant improvement in clinical outcomes when compared with placebo.56) Another PDE3 inhibitor, milrinone, and enoximone have been investigated for the treatment of advanced HFrEF. However, the use of milrinone increased all-cause mortality by 28% and CV mortality by 34% when compared with placebo. In addition, the risks of hospitalization and adverse events were higher in the milrinone group.57) The use of enoximone did not improve clinical outcomes in patients with NYHA class II–III HFrEF compared with placebo.88) Similar results were reported in the ESSENTIAL trial, which included more patients with NYHA class III–IV than previous trials.58) A study with vesnarinone, a PDE3 inhibitor and a ion-channel modifier, resulted in a dose-dependent increase in mortality among patients with advanced HF.59) Consequently, PDE inhibitors are currently only used in patients with acute decompensated HF.89)
Calcium sensitizers
Calcium sensitizers increase myocardial contractility by retaining the calcium-binding site of troponin C in an active form, even under low calcium conditions.90) Pimobendan is a calcium sensitizer and PDE3 inhibitor. These agents were expected to exert a positive effect on HF by strengthening the heart muscle and relaxing the peripheral blood vessels. In the PICO trial, the effects of placebo and pimobendan were compared in patients with HFrEF. However, in the pimobendan group, both all-cause death and death or first hospitalization were higher, compared with the placebo group.60) Studies have also been performed using levosimendan, another calcium sensitizer. In the LevoRep trial, the effects of levosimendan and placebo were compared in patients with advanced HF and shown to improve functional capacity and quality of life. However, levosimendan did not significantly reduce event-free survival compared with placebo.61) In the LION-HEART trial, levosimendan was compared with placebo in a relatively small number of patients. Sixty-nine patients with HFrEF were treated with levosimendan via a 6-hour intravenous infusion every 2 weeks for 12 weeks. Levosimendan significantly reduced N-terminal pro-B-type natriuretic peptide (NT-proBNP) and hospitalization in patients with HF compared with placebo, but did not improve mortality.62) A larger trial may be required in future.
Cardiac glycosides
Digoxin is a cardiac glycoside that has an inotropic effect and has been widely used in patients with CHF. In the DIG trial, the effects of digoxin and placebo were compared in 3,397 patients (ejection fraction ≤45%) receiving standard treatment, including ACEI and diuretics. During the follow-up period of an average 37 months, there was no significant difference in all-cause mortality between groups. However, digoxin therapy reduced the incidence of death attributed to worsening HF (relative risk [RR], 0.88) and hospitalization due to worsening HF (RR, 0.72).63) Currently, digoxin is prescribed for patients with advanced HF to improve symptoms.43)
DRUGS WITH A VASODILATORY EFFECT
Calcium channel blockers (CCBs)
CCBs exacerbate chronic HF due to negative inotropic effects and neuroendocrine activation. Despite this, CCBs have been investigated for their potential use as vasodilators in patients with CHF. When vasodilators such as H-ISDN were a focus of HF treatment, the survival benefits of some first-generation CCBs were assessed in patients with HFrEF when added to conventional therapy.91) A study evaluating the effectiveness of a first-generation CCB, nifedipine, divided patients into four groups: placebo, nifedipine, isosorbide dinitrate (ISDN), and combined nifedipine and ISDN. When either nifedipine or combination therapy was used, patients experienced more hospitalization and worse episodes of HF than patients in the ISDN group.64) In the DAVIT-II trial, verapamil failed to show clinical benefits in patients with HF.65) Similarly, a study comparing diltiazem and placebo in post-infarction patients with LV dysfunction did not show any beneficial effects, but rather increased the risk of CHF.66)
The second-generation CCBs, amlodipine, and felodipine have little or no negative inotropic activity at the usual therapeutic doses. Based on this, the PRAISE-I and PRAISE-II trials compared the effects of amlodipine addition in patients with HFrEF. Amlodipine did not increase CV morbidity or mortality, or demonstrated any favorable effects compared with placebo.67)68) Felodipine was also compared with placebo in patients with CHF. The drug was considered safe, and increased exercise tolerance and quality of life; however, no improvement in survival was reported.69) Most CCBs are not recommended for clinical use in the guidelines. Only amlodipine and felodipine are recommended for use in limited indications.1)
Dopamine
Ibopamine is an orally active dopamine agonist that activates DA-1 and DA-2 receptors. Dopamine agonists induce renal and peripheral vasodilation, and has a minimal inotropic effect at low doses. Based on this, dopamine was used in the early days of HF treatment, based on the belief that it caused decongestion and preserved renal function. In the PRIME-II trial, ibopamine was compared with placebo in patients with advanced HFrEF. However, the trial was stopped early because of higher mortality in the ibopamine group (RR, 1.26).70) Currently, dopamine is used in some patients with acute HF who have low blood pressure; however, the evidence for long-term treatment remains insufficient.89)
Endothelin (ET) receptor antagonists
ET receptors contribute to blood vessel constriction by binding to ET-1 and causing the proliferation of vascular smooth muscle and cardiac hypertrophy. ET receptors are divided into ETA and ETB, and the former subtype is mainly responsible for vasoconstriction in response to ET-1. In addition, reports have indicated that ET-1 is elevated in patients with chronic HF.92) Thus, the endothelin receptor antagonist (ERA) has been used as a treatment for HF via vasodilation and reversal of myocardial hypertrophy. The effectiveness of a non-selective ETA/ETB ERA, darusentan, in patients with HFrEF was compared with placebo in the EARTH trial. Mortality was not investigated in this study, and no improvements in LV end-systolic volume or symptoms were reported.71) The effect on morbidity and mortality was confirmed in the ENABLE trial, which was conducted with bosentan, an ETA receptor-selective ERA. However, in patients with advanced HFrEF, there was no difference in the primary outcome (death from any cause or hospitalization for HF) between bosentan and control groups. In addition, patients in the bosentan group experienced more peripheral edema or weight gain at baseline.72)
Quinolone vasodilators
Flosequinan is categorized as a quinolone vasodilator with peripheral arteriolar and venous vasodilatory effects. In addition, it increases intracellular calcium levels, resulting in positive inotropic and chronotropic effects, which differ from the effects of other vasodilators or inotropes. The effects of flosequinan were demonstrated in the PROFILE trial, which enrolled patients with advanced HFrEF. However, the trial was stopped due to safety concerns after a higher number of deaths were reported in the flosequinan group (HR, 1.39).73)
TARGETING OTHER MOLECULES IN THE RAAS
Renin inhibitors
Direct renin inhibitors (DRIs) are novel upstream RAAS inhibitors. Aliskiren is a first-inclass, orally active DRI that is approved for the treatment of hypertension.93) Several clinical trials have investigated aliskiren in patients with HF. For example, the ALOFT trial compared the effects of aliskiren with placebo in patients with HF receiving standard treatment, such as ACEI and BB. Aliskiren was well tolerated by patients and succeeded in lowering plasma NT-proBNP, plasma renin activity, and urinary aldosterone excretion. However, it did not improve symptoms or other important clinical indicators.74) In the ASTRONAUT trial, clinical outcomes in patients with HFrEF were compared following treatment with aliskiren or placebo. The results showed that aliskiren increased the rate of hyperkalemia, hypotension, and renal dysfunction, and did not reduce CV death or HF rehospitalization when compared with placebo.75) In the ATMOSPHERE trial, patients were divided into 3 groups: aliskiren and enalapril, or a combination of aliskiren and enalapril. In patients with HFrEF and high BNP levels, there was no difference in the primary outcome (a composite of death from CV causes or hospitalization for HF); however, the pre-specified criterion for non-inferiority was not met. Combination therapy was reported to increase the occurrence of adverse events, such as hypotension, hyperkalemia, and serum creatinine elevation when compared with enalapril.76)
DRUGS WITH OTHER MECHANISMS
Antithrombotic drugs
Patients with HF have a high probability of sudden death from thromboembolic events such as MI or stroke. Arrhythmias, such as atrial fibrillation, lead to a higher risk of such events.94) However, it was unclear whether all patients with HF should receive antithrombotic therapy. Considering this, several RCTs have been conducted with antithrombotic drugs.
The WASH trial compared placebo, aspirin, and warfarin in patients with HFrEF who had no other indication for aspirin or warfarin. Patients with atrial fibrillation did not account for a large proportion of the study population (4% in the placebo group, 7% in the aspirin group, and 7% in the warfarin group). Although all-cause hospitalization was higher in the aspirin group compared with the placebo and warfarin groups, there were no differences in other clinical outcome.77) In the HELAS trial, placebo and aspirin were administered to patients with HFrEF and ischemic heart disease, and placebo and warfarin were administered to patients with HFrEF and dilated cardiomyopathy. In addition, there was no significant correlation between treatment and clinical outcome.78) The WATCH trial was conducted in patients with HFrEF patients and sinus rhythm. Aspirin, clopidogrel, and warfarin were compared, with no difference in mortality reported between the 3 groups.79) Similarly, there were no differences in clinical outcomes when comparing aspirin and warfarin in the WARCEF trial in patients with sinus rhythm and HFrEF.80)
Antithrombotic drugs have not be found to reduce mortality in patients with LV dysfunction and sinus rhythm; however, the development of factor-Xa inhibitors has raised expectations. In the COMMANDER HF trial, rivaroxaban, a factor Xa inhibitor, was compared with placebo in patients with HF and coronary artery disease, ejection fraction less than 40%, and no atrial fibrillation. No statistically significant benefit was reported in the composite of death from any cause, myocardial infarction, or stroke, which was the primary outcome (HR, 0.94; 95% confidence interval, 0.84–1.05; p=0.27).81)
Statins
Statins are prescribed widely for patients with CV disease, especially in those with coronary artery disease, for the prevention of MI. However, the effectiveness of statins in patients with HF has not been well proven. Statins may help HF by improving endothelial function owing to their anti-inflammatory properties. However, they may also induce cardiac myopathy by inhibiting the synthesis of coenzyme Q10 and selenoproteins. The CORONA trial compared rosuvastatin and placebo in elderly patients with HFrEF. Compared with placebo, the levels of high-sensitivity C-reactive protein (hsCRP) and low-density lipoprotein cholesterol were lower in the rosuvastatin group. No safety concerns were observed, and the rosuvastatin group had fewer hospitalization for CV disease. However, there was no difference in mortality between the 2 groups when compared with placebo.82) The GISSI-HF trial, which was conducted in a large population of patients with HFrEF aged 18 years or older, also compared rosuvastatin and placebo. Similarly, there was no significant difference in clinical outcomes compared with placebo.83)
Anti-cytokine drugs
Owing to a more detailed understanding of HF pathophysiology, the importance of inflammation on ventricular remodeling in HF has become evident, through mechanisms such as immunosenescence and age-related changes in the immune system.95) Several attempts have been made to reduce the mortality of patients with HF using anti-inflammatory agents. The first attempt to modulate the immune system involved the use of prednisone.96) Drugs used for the treatment of gout, such as colchicine97) and allopurinol,98) were subsequently evaluated; however, these treatments did not have a significant effect on clinical outcomes. Eventually, drugs targeting pro-inflammatory cytokines were assessed in patients with HFrEF.
Previous studies have investigated tumor necrosis factor (TNF)-α inhibitors. The ATTACH trial focused on infliximab,84) a chimeric monoclonal antibody against TNF-α, and the RENEWAL85) trial targeted etanercept, a recombinant human TNF receptor that binds to circulating TNF. These anti-inflammatory drugs were compared with placebo in patients with advanced HFrEF. However, the results showed no risk reduction on mortality or hospitalization. Recently, a large RCT was conducted with an interleukin-1β inhibitor, canakinumab. In the CANTOS trial, patients were randomly assigned to treatment with canakinumab 50, 150, 300 mg, or placebo. The study enrolled 10,061 patients with prior history of MI and hsCRP >2 mg/L. In the canakinumab group, there was a trend for a dosedependent reduction in hospitalization for HF and a composite of hospitalization for HF or HF-related mortality. However, these results were not statistically significant in patients who initially had a history of CHF.86) Despite these findings, the CANTOS trial provided possibilities for the identification of novel treatments for HFrEF.
CONCLUSION
In patients with HFrEF, pharmacological treatments involving neurohormonal blockade have demonstrated substantial success. However, in phase III clinical trials, many drugs demonstrated no significant improvement in primary endpoints, despite the potential benefits found in pilot studies and based on the underlying mechanisms of action. These efforts have led to a better understanding of HFrEF pathophysiology and have provided insights into the identification of new drug targets. Several novel drugs and interventions are under evaluation in patients with HFrEF. The novel pharmacotherapy for HFrEF may open a new paradigm for improving the outcome of these patients.
Notes
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2018R1C1B6005448). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors have no financial conflicts of interest.
Author Contributions
Conceptualization: Kim ES; Supervision: Youn JC; Validation: Baek SH; Writing - review & editing: Kim ES.