Calcium channel blockers for hypertension: old, but still useful
Article information
Abstract
Calcium channel blockers (CCBs) constitute a heterogeneous class of drugs that can be divided into dihydropyridines (DHPs) and non-DHPs. DHP-CCBs are subcategorized into four generations based on the duration of activity and pharmacokinetics, while non-DHP-CCBs are subcategorized into phenylethylamine and benzodiazepine derivatives. DHP-CCBs are vascular-selective and function as potent vasodilators, whereas non-DHP-CCBs are cardiac-selective and are useful for treating tachyarrhythmia, but reduce cardiac contractility and heart rate. Traditional DHP-CCBs (nifedipine) mainly block L-type calcium channels, whereas novel CCBs block N-type (amlodipine) and/or T-type channels (efonidipine) in addition to L-type channels, leading to organ-protective effects. DHP-CCBs have a potent blood pressure–lowering effect and suppress atherosclerosis and coronary vasospasm. Diltiazem, a non-DHP-CCB, is highly effective for vasospasm control. CCBs reduce left ventricular hypertrophy and arterial stiffness. Amlodipine, a DHP-CCB, reduces blood pressure variability. L/N- and L/T-type CCBs combined with renin-angiotensin system blockers reduce proteinuria and improve kidney function compared with L-type CCBs. According to large-scale trials, DHP-CCBs reduce cardiovascular events in patients with isolated systolic hypertension, as well as in elderly and high-risk patients. Accordingly, CCBs are indicated for hypertension in elderly patients, isolated systolic hypertension, angina pectoris, and coronary vasospasm. Non-DHP-CCBs are contraindicated in high-grade heart block, bradycardia (<60 beats per minute [bpm]), and heart failure with reduced ejection fraction (HFrEF). DHP-CCBs should be used with caution in patients with tachyarrhythmia, HFrEF, and severe leg edema, and non-DHP-CCBs should be used carefully in those with constipation. Each CCB has distinct pharmacokinetics and side effects, underscoring the need for meticulous consideration in clinical practice.
INTRODUCTION
Calcium channel blockers (CCBs) play a crucial role in the treatment of hypertension, either as an initial monotherapy or in combination with other classes of antihypertensive drugs in Korea [1]. Beyond blood pressure (BP) control, they are also utilized for conditions such as angina pectoris, coronary vasospasm, and arrhythmias [2]. These medications constitute a heterogeneous class of drugs that can be divided into dihydropyridines (DHPs) and non-DHPs according to the main site of action, each with distinct pharmacokinetic profiles and side effects [3–6].
This review provides an overview of CCBs: (1) their classification and pharmacokinetic profiles; (2) the underlying mechanism of action; (3) their efficacy in lowering BP and reducing BP variability; (4) their role in protecting against target organ damage; (5) their potential for preventing cardiovascular events; and (6) the indications, contraindications, and side effects associated with CCBs.
CLASSIFICATION AND PHARMACOKINETIC PROFILES
CCBs are categorized into DHP-CCBs and non-DHP-CCBs based on their chemical structure (Fig. 1) [3–6]. DHP-CCBs primarily act on vascular smooth muscles, promoting vasodilation without significantly affecting cardiac function (vascular selectivity). These drugs are further divided into four generations based on their discovery time, onset of drug effect, and duration of activity. Long-acting CCBs are characterized by higher vascular selectivity, increased lipophilicity, and reduced sympathetic excitation. Conversely, non-DHP-CCBs exert a more pronounced effect on the conduction system and ventricular muscle, but are less effective in promoting vasodilation (cardiac selectivity). These drugs are subcategorized as phenylethylamine (PAA) and benzodiazepine (BTZ) derivatives based on their chemical structure. Each category of non-DHP-CCBs is further divided into two generations according to the duration of action. Representative DHP-CCBs include the following: first-generation CCBs such as short-acting nifedipine and nicardipine, which have a rapid onset and short duration of vasodilating activity; second-generation drugs such as extended-release nifedipine, felodipine, benidipine, and efonidipine, which have a slow release and short duration of activity; third-generation drugs such as amlodipine and azelnidipine, which exhibit stable pharmacokinetics (e.g., slow action and long duration of activity), higher vascular selectivity, and less sympathoexcitation, resulting in less cardiac selectivity and thus, better tolerance in patients with heart failure (HF); and fourth-generation drugs including lacidipine, lercanidipine, and cilnidipine, which have stronger lipophilicity, leading to stable activity, reduction in peripheral edema, and a broad therapeutic spectrum, especially for myocardial ischemia and HF [4,7]. The first-generation short-acting nifedipine was associated with increased mortality and myocardial ischemia due to rebound activation of sympathetic activity caused by its short duration and rapid onset of vasodilating activity [4]. Therefore, it is currently not recommended by the 2022 Korean Society of Hypertension (KSH) Guideline [2] and is not commercially available in Korea. Among non-DHP-CCBs, PAA derivatives, such as verapamil, exhibit higher cardiac selectivity, acting on the impulse conduction system (ICS) including the sinoatrial (SA) node and atrioventricular (AV) node, and ventricular muscle. As a result, it has negative inotropic, chronotropic, and dromotropic effects; thus, it is useful for arrhythmia treatment, but it should be avoided in cases of HF and bradycardia [2,3,8]. BTZ derivatives, such as diltiazem, have an intermediate effect between DHP-CCBs and PAA derivatives, acting on the myocardium and ICS, particularly in the AV node. These drugs have a very strong effect on coronary vasodilation, making them useful for treating coronary vasospasm [3]. Representative non-DHP-CCBs are as follows: first-generation, verapamil and diltiazem; and second-generation, verapamil sustained release and diltiazem sustained release [3]. The pharmacokinetic profile of each CCB is presented in Table 1 [3,4,6,7,9–17].

Classification and pharmacological actions of calcium channel blockers (CCBs). DHP, dihydropyridine; PAA, benzodiazepine; Non-DHP, non-dihydropyridine; SR, sustained release; ↑↑↑, strongest; ↑↑, very strong; ↑, strong positive action; →, neutral action; ↓, negative action.
Based on data from Sueta et al. [3], and Wang et al. [4], Elliott et al. [5], Wang et al. [6].
MECHANISMS
The first study of CCBs was reported by Fleckenstein et al. [18] in 1969. CCBs disrupt the inward movement of extracellular calcium (Ca2+) through the calcium channel, leading to a decrease in peripheral vascular resistance and, consequently, reduced BP [4]. Additionally, CCBs induce coronary vasodilation and inhibit ventricular contraction and the intracellular signaling system (ICS), leading to anti-anginal and anti-arrhythmic effects (Fig. 2) [3]. Voltage-gated Ca2+ channels are composed of four subunits: α1 and α2, δ, β, and γ. These channels are pharmacologically classified into different subtypes (Fig. 3A) [19,20]: high voltage–activated (L- and N-type), low voltage–activated (T-type), and P/Q- and R-types. The characteristics of these channels are determined by the pore-forming α1 subunit. Traditional CCBs, such as nifedipine and felodipine, primarily affect L-type channels, acting as potent vasodilators. In contrast, novel CCBs influence N-type (cilnidipine, amlodipine) and/or T-type channels (efonidipine) in addition to L-type channels. N-type channels are associated with decreased norepinephrine release at sympathetic nerve endings, while T-type channels are linked to improved renal microcirculation (Fig. 3B) [11,12,21]. Therefore, it is hypothesized that the combined blocking of N- or T- channels, in addition to L-type channels by CCBs, may exert organ-protective actions in the treatment of hypertension, beyond just lowering BP. Furukawa et al. [10] demonstrated the selectivity of DHP-CCBs for calcium channel subtypes, suggesting that these properties could provide different pharmacological information and influence the adverse effects of DHP-CCBs.
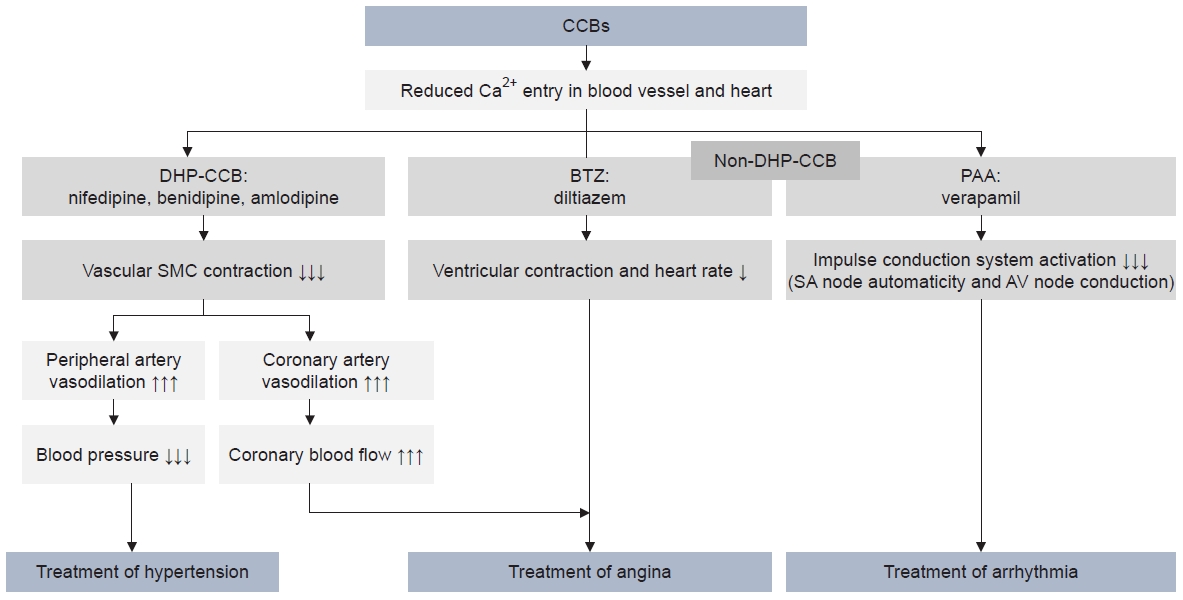
Three main pharmacological mechanisms of calcium channel blockers (CCBs): treatment of hypertension through peripheral vasodilation; treatment of angina pectoris through coronary artery vasodilation and decreased ventricular contraction and heart rate; and arrhythmia treatment through decreased impulse conduction system excitation. Downward arrow indicate negative action, upward arrow indicate positive action (the degree is presented by the number of arrows). DHP, dihydropyridine; BTZ, benzothiazepine; PAA, phenylalkylamine; SMC, smooth muscle cell; SA node, sinoatrial node; AV node, atrioventricular node.
Based on data from Sueta et al. [3].
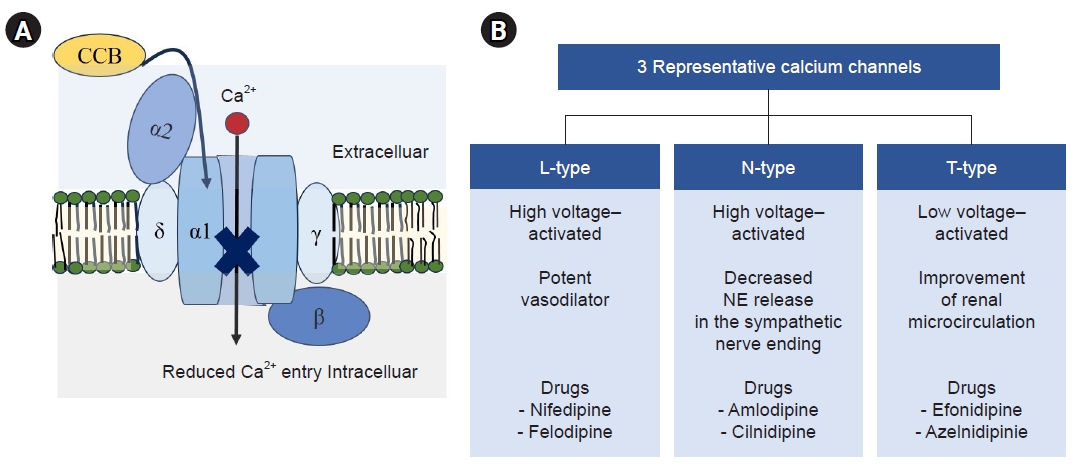
Voltage-gated calcium channels and three representative types. (A) Voltage-gated calcium channels. Voltage-gated extracellular calcium (Ca2+) channels consist of four subunits, α1 and α2, δ, β, and γ, and they are pharmacologically classified into different subtypes; the characteristics of which are determined by the pore-forming α1 subunit. Calcium channel blockers (CCBs) disrupt the inward movement of Ca2+ through the calcium channel. (B) Three representative types of calcium channel. Calcium channels are pharmacologically classified into different subtypes: high voltage–activated (L- and N-type) and low voltage–activated (T-type). L-type channels act as potent vasodilators, N-type channels have decreased norepinephrine (NE) release in the sympathetic nerve ending, and T-type channels have improvement of renal microcirculation. It is speculated that the combined blocking of N- or T-channels in addition to traditional L-type blocking in CCBs leads to different pharmacologic impacts and adverse effects of dihydropyridine CCBs.
EFFICACY OF BP REDUCTION
Wang et al. [6] found that DHP-CCBs demonstrated a superior 24-hour BP reduction compared to other classes of antihypertensive drugs, including renin-angiotensin system (RAS) blockers, β-blockers, and diuretics. The weighted mean difference was 5 mmHg for systolic BP and 3 mmHg for diastolic BP. Furthermore, in the DHP-CCB group, both daytime and nighttime systolic BP reductions were significantly and positively correlated with the BP value at baseline. This correlation was weak and not statistically significant in other drug classes. A Cochrane review [22] also revealed a relatively consistent BP-lowering effect at each hour over a 24-hour period among six DHP-CCBs, nifedipine, felodipine, manidipine, amlodipine, lercanidipine, and nicardipine. Lorimer et al. [23] reported that amlodipine had better BP reduction than lisinopril (supine systolic/diastolic BP reduction 24-hour after dosing, –12%/–14% decrease in amlodipine group vs. –7%/–7% decrease in lisinopril group). Additionally, amlodipine provided more consistent control of BP over 24 hours compared to lisinopril, due to the significantly longer half-life of amlodipine (35–50 hours) compared to lisinopril (12.6 hours). Thus, DHP-CCBs have a potent BP-lowering effect.
BP VARIABILITY
Rothwell et al. [24] found that in patients with treated hypertension, variability in systolic BP was linked to vascular events, independent of the mean systolic BP. In the ASCOT-BPLA (Anglo-Scandinavian Cardiac Outcomes Trial Blood Pressure Lowering Arm) Study [25], the variability of systolic BP was lower in the group treated with amlodipine compared to the group treated with atenolol. Furthermore, subsequent trends in BP variability during follow-up in the atenolol group were associated with trends in stroke risk. This finding partially explains the reduced risk of vascular events observed in the amlodipine group. In a meta-analysis [26], it was found that CCBs and nonloop diuretics reduced interindividual systolic BP variability, while RAS blockers and β-blockers increased it. The ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) Study [27] suggests that chlorthalidone and the DHP-CCB amlodipine are associated with lower systolic BP variability than lisinopril.
PROTECTION FOR HMOD
LVH and HF
CCBs provide protective effects against hypertension-mediated organ damage (HMOD). Different classes of antihypertensive drugs have varying impacts on the reduction of left ventricular hypertrophy (LVH) [28]. Klingbeil et al. [29] reported that LVH decreased by 13% with angiotensin receptor blockers (ARBs), 11% with CCBs, 10% with angiotensin-converting enzyme inhibitors (ACEIs), 8% with diuretics , and 6% with β-blockers. Therefore, CCBs serve as an intermediate-range solution for LVH reduction and are recommended for treating hypertensive patients with LVH. However, CCBs are less effective than other first-line antihypertensive drugs in protecting against HF [30]. The ALLHAT Study [31] aimed to determine whether treatment with CCBs or ACEIs reduces the incidence of coronary artery disease (CAD) or other cardiovascular disease (CVD) events compared to treatment with diuretics. The study found no difference in primary outcomes between treatment groups, but HF was higher in the amlodipine group. Conversely, the PRAISE-2 (Prospective Randomized Amlodipine Survival Evaluation 2) Study [15] showed that amlodipine had a neutral effect on mortality based on ejection fraction. According to the 2023 Guidelines from the European Society of Hypertension (ESH) [8], in patients with hypertension and HF with reduced ejection fraction (HFrEF), the initial recommendation is to combine drugs with sacubitril/valsartan, RAS blockers, β-blockers, aldosterone inhibitors, and sodium-glucose cotransporter 2 (SGLT2) inhibitors. If control is not achieved, a DHP-CCB can be added for BP control. The use of non-DHP-CCB is not recommended in HFrEF due to their negative inotropic effects. For patients with hypertension and HF with preserved ejection fraction, the treatment of hypertension with all major antihypertensive drugs, including CCBs, is recommended.
Arterial stiffness
Increased arterial stiffness, as measured by pulse wave velocity (PWV), is a potent independent predictor of cardiovascular morbidity and mortality in patients with hypertension [32]. Therefore, reducing arterial stiffness can lower cardiovascular risk. However, the data on the impact of CCBs on arterial stiffness is limited, though some studies suggest beneficial outcomes. For instance, Hayoz et al. [33] reported that treatments with amlodipine and valsartan for 38 weeks similarly reduced carotid-femoral PWV in postmenopausal women with hypertension. Takami and Shigemasa [34] found that ARBs resulted in the most significant reductions in pulse pressure and brachial PWV. This was followed by ACEIs and L- and N-type CCBs, while L-type CCBs showed no improvement. Matsui et al. [35] demonstrated that a combination of ARB (olmesartan, 20 mg) and DHP-CCB (azelnidipine, 16 mg) had a more beneficial effect on central systolic BP and arterial stiffness than the combination of ARB and diuretic, despite similar reductions in brachial systolic BP between the two treatments. Thus, DHP-CCB monotherapy or combination therapy with a DHP-CCB and ARB might protect against arterial stiffness
Atherosclerosis and coronary vasospasm
Henry and Bentley [36] conducted an experiment to test the impact of nifedipine on atherosclerosis in rabbits fed a cholesterol diet. Their findings showed that aortic lesions stainable with Sudan IV covered 40%±5% of the intimal surface in animals in the placebo group versus 17.3%±3% in the nifedipine group. This finding means lesion formation for a given lipid accumulation was significantly reduced in nifedipine-treated rabbits. However, the total cholesterol concentration was 48±7 mg/dL in the placebo group versus 46±6 mg/dL in the nifedipine group. These results suggest that nifedipine can suppress atherogenesis without reducing hypercholesterolemia. The PREVENT (Prospective Randomized Evaluation of the Vascular Effects of Norvasc Trial) Investigation [37] reported that amlodipine does not have a noticeable effect on the angiographic progression of coronary atherosclerosis or the risk of major CVD events. However, it is associated with fewer hospitalizations due to unstable angina and revascularization. The ELSA (European Lacidipine Study on Atherosclerosis) Trial [38] found that lacidipine, a DHP-CCB, had a greater effect on the progression of carotid intimal-medical thinness and the number of plaques per patient. Despite a smaller reduction in ambulatory BP, this suggests an anti-atherosclerotic action of this drug. Ishii et al. [39] proposed that the anti-atherosclerotic effect of DHP-CCBs is achieved by suppressing the generation of reactive oxygen species, the expression of adhesion molecules, and the progression and migration of smooth muscle cells. In macrophages, they reduce cholesterol accumulation and suppress the expression of matrix metalloproteinases, as well as activate peroxisome proliferator-activated receptor-γ. Furthermore, CCBs are highly effective in suppressing coronary vasospasm. Nishigaki et al. [40] showed that among four CCBs (benidipine, amlodipine, nifedipine, and diltiazem), major CVD events in patients with vasospastic angina were significantly lower in patients treated with benidipine. Recently, Kim et al. [41] reported that there was no difference in cardiovascular outcome occurrence in patients with vasospastic angina treated with first-generation CCBs (including diltiazem and nifedipine) and second-generation CCBs (including amlodipine and benidipine). However, the incidence rate of acute coronary syndrome was significantly lower in patients treated with second-generation CCBs. According to the 2023 ESH Guidelines [8], in patients with hypertension and CAD with angina pectoris, both DHP and non-DHP-CCB are particularly useful, and β-blockers should not usually be combined with non-DHP-CCB. Hypertension and LVH are often associated with myocardial ischemia and nonobstructive CAD. In such cases, treatment with CCBs can be beneficial.
Renal protection
Reducing albuminuria or proteinuria is a crucial surrogate goal in hypertension treatment, as it helps decrease both chronic kidney disease (CKD) and CVD. To achieve the target goal in CKD, a combination therapy is typically required, involving a RAS blocker with a CCB or a diuretic, particularly if estimated glomerular filtration rate (eGFR) levels are at CKD stages ≥3a [8]. A secondary analysis of the ACCOMPLISH (Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension) Study [42,43] demonstrated the superior efficacy of combining benazepril and amlodipine over benazepril plus hydrochlorothiazide, as it slows nephropathy progression. Therefore, CCBs are generally recommended as the drug to combine with RAS blockers in high-risk patients, as this combination therapy effectively reduces BP and primary protein excretion [8]. However, the kidney protection mechanism of CCBs may vary depending on the types of calcium channels. L-type CCBs increase glomerular pressure through dilation of the afferent artery and constriction of the efferent artery. In contrast, L/N-type and L/T-CCBs decrease glomerular pressure and improve glomerular microcirculation through vasodilatory activity on both afferent and efferent arterioles [9,12,44]. Thamcharoen et al. [16] found that L/N-CCB (cilnidipine) and L/T-type CCBs (azelnidipine, efonidipine, and benidipine) combined with RAS blockers resulted in a decrease in proteinuria and an improvement in kidney function compared to standard L-type CCBs, despite no additional BP-lowering effect. Lercanidipine, an L/T channel blocker, is highly lipophilic compared to amlodipine and directly dilates both the afferent and efferent glomerular arteries without altering intraglomerular capillary pressure [17]. Burnier [45] reported that lercanidipine appears to provide renal protection similarly to an ACEI, while amlodipine is generally less effective in terms of renal protection. The beneficial effects of amlodipine in slowing the progression of renal disease are only achievable when combined with a RAS blocker. Zhao et al. [46] reported that combined treatment with RAS blockers and L/T-type CCBs reduced proteinuria without increasing kidney function and adverse effects. This finding is independent of BP and may be associated with decreased aldosterone.
PREVENTION OF CARDIOVASCULAR EVENTS
DHP-CCBs have been extensively studied for the prevention of CVD, in comparison to various classes of antihypertensive drugs. The Sys-Eur (Systolic Hypertension in Europe) Trial [47] was a randomized, double-blind comparison of a placebo and active treatment with a CCB (nitrendipine) for older patients (>60 years, n=4,695) with isolated systolic hypertension (ISH). The group receiving active treatment with CCBs experienced a reduction in BP of 10.1 mmHg in systolic BP and 4.5 mmHg in diastolic BP, compared to the placebo group. Furthermore, active treatment led to a decrease in the rate of cardiovascular complications; total stroke was reduced by 42% (P=0.003), fatal and nonfatal cardiac endpoints by 31% (P=0.03), and cardiovascular mortality by 27% (P=0.07). The INSIGHT (Intervention as a Goal in Hypertension Treatment) Study [48] compared the effects of nifedipine, a CCB, with the diuretic combination co-amilozide on cardiovascular mortality and morbidity in high-risk patients with hypertension. The overall mean BP dropped from 173/99 mmHg to 138/82 mmHg. Nifedipine once daily and co-amilozide were equally effective in preventing overall cardiovascular or cerebrovascular complications. Based on the above studies, DHP-CCBs are recommended in elderly patients with hypertension and those with ISH [49]. The VALUE (Valsartan Antihypertensive Long-term Use Evaluation) Trial [50] was designed to test the hypothesis that valsartan would reduce cardiac morbidity and mortality more than amlodipine in hypertensive patients at high CVD risk. However, contrary to expectations, the results showed that the amlodipine-based regimen had a more pronounced BP-lowering effect than the valsartan-based regimen, especially during the first 6 months (BP was 4.0/2.1 mmHg lower in the amlodipine group than in the valsartan group after 1 month, 1.5/1.3 mmHg after 1 year; P<0.001 between groups). However, the primary composite endpoint was similar between the two regimens. Myocardial infarction occurred less frequently in the amlodipine group (4.1% vs. 4.8%; hazard ratio [HR], 1.19; P=0.02), and stroke trended lower (3.7% vs. 4.2%; HR, 1.15; P=0.08). New onset diabetes was higher in the amlodipine group (16.4% vs. 13.1%; HR, 0.77; P<0.0001), and HF trended higher (5.3% vs. 4.2%; HR, 0.89; P=0.12). This study emphasized that BP reduction is more important than the mechanism of action of the drug, and that amlodipine-based treatment has a powerful BP-lowering effect in the early phase. Therefore, the quicker patients can reach the target with CCBs, the better the protection they will receive. In the ASCOT-BPLA Study [25], patients were randomized to one of two BP-lowering treatments, either amlodipine with or without perindopril (amlodipine-based) or atenolol with or without bendroflumethiazide (atenolol-based). The study found that the amlodipine-based regimen prevented more major CVD events and induced less diabetes than the atenolol-based regimen. Furthermore, the ASCOT Legacy Study [51], results after a 16-year follow-up, showed that significantly fewer deaths from stroke occurred in the amlodipine-based treatment group than in the atenolol-based treatment group. Patients with combined treatment with lipid-lowering treatment had fewer CVD deaths more than 10 years after the trial closure. These studies have important implications, suggesting that combined interventions with BP-lowering CCBs and lipid-lowering treatments are associated with long-term benefits in patients with hypertension and no history of CAD events. The ACCOMPLISH Study [39] aimed to investigate the efficacy of the combination treatment of an ACEI and DHP-CCB in reducing the rate of CVD events, compared to treatment with an ACEI plus a thiazide diuretic in high-risk patients with hypertension. The combination of benazepril and amlodipine was found to be superior to the combination of benazepril and hydrochlorothiazide in reducing CVD events in high-risk patients. Therefore, the aforementioned studies suggest that DHP-CCBs can effectively reduce CVD events in ISH, elderly, and high-risk patients. Moreover, these agents, when combined with RAS blockers, can be effective in reducing CVD events.
INDICATIONS, CONTRAINDICATIONS, AND SIDE EFFECTS
CCBs are indicated for a variety of conditions, including hypertension in the elderly, ISH, angina pectoris, and coronary vasospasm, according to the 2022 KSH Guidelines [2]. Additionally, non-DHP-CCBs such as verapamil and diltiazem are suggested for use post–myocardial infarction, as they do not cause rebound tachycardia. They are also recommended for hypertrophic cardiomyopathy due to their ability to improve diastolic filling time. The common side effects of DHP-CCBs include tachycardia, peripheral edema, headaches, and hot flashes, while non-DHP-CCBs may cause constipation and bradycardia [5]. Non-DHP-CCBs are contraindicated in cases of high-grade SA or AV block, HFrEF, bradycardia (for instance, a heart rate of less than 60 bpm), and when comedications are susceptible to significant drug interactions mediated by P-gp or CYP3A4. DHP-CCBs should be used cautiously in cases of tachyarrhythmia, HFrEF (class III or IV), and preexisting severe edema. Similarly, non-DHP-CCBs should be used with caution in cases of constipation [8].
Peripheral edema
There is no universally accepted definition of peripheral edema related to CCBs, which results in a wide range of reported incidence rates, from 5% to 70% [52]. This condition is more prevalent in women, in individuals who are often in an upright position, and in the elderly. The incidence is 2.8 times higher with high-dose CCBs than with low-dose CCBs [52,53]. Liang et al. [53] found that the incidence of peripheral edema was significantly higher with DHP-CCBs than with non-DHP-CCBs. Among DHP-CCBs, the first-generation CCB nifedipine had the highest incidence of peripheral edema, while the fourth-generation CCB lacidipine had the lowest incidence, according to the systematic review and network meta-analysis. The COHORT Study [54] revealed that amlodipine was associated with significantly more edema-related symptoms (19%) than lipophilic CCBs, lercanidipine (9%) or lacidipine (4%). The primary mechanism behind this is postulated to be an imbalance between precapillary and postcapillary tones, leading to intracapillary hypertension and fluid leakage. The rate of drug withdrawal due to edema was 2.1 times higher in the CCB group than in the placebo group [55]. Therefore, it is crucial to identify and manage peripheral edema when treating patients with CCBs. Treatment strategies for CCB-related edema may include the following: (1) reducing the dose of CCBs; (2) switching from a DHP-CCB to a non-DHP-CCB or to a lipophilic CCB such as lercardipine or lacidipine; (3) coadministration of RAS blockers; and (4) diuretic therapy. Diuretics, particularly thiazide diuretics, have been suggested as a treatment option for edema due to their ability to decrease limb volume. However, since CCB-related edema is not associated with volume overload at a fundamental level, routine administration of diuretics is not recommended for patients with edema to reduce the edematous state [52]. Vouri et al. [56] reported that a significant proportion of patients were prescribed loop diuretics instead of having their DHP-CCB dose reduced or discontinued. This led to adverse events associated with loop diuretics in the first 4 months following initiation, highlighting the need to evaluate edema after starting CCBs. The combination of DHP-CCB and RAS blockers has been suggested to reduce edema compared to DHP-CCB monotherapy due to the balanced vasodilating effect of RAS blockers on both precapillary and postcapillary tones. For instance, a combination of amlodipine and an ACEI was found to reduce edema the most, while a combination of nifedipine and an ARB did not alleviate edema, although information on this is limited [52,53]. Therefore, the use of long-acting and lipophilic DHP-CCBs in combination with RAS blockers may decrease the likelihood of peripheral edema development compared to DHP-CCB monotherapy [53].
CONCLUSIONS
This review provides an overview of CCBs. The following major points are highlighted.
First, CCBs are categorized into two types: DHP-CCBs and non-DHP-CCBs. DHP-CCBs are further subcategorized into first- to fourth-generation based on their time of discovery, onset of drug effect, and duration of activity. They exhibit higher vascular selectivity, making them potent vasodilators. Conversely, non-DHP-CCBs have higher cardiac selectivity, making them suitable for treating tachyarrhythmia, although they should be used with caution in cases of HF and bradycardia. Traditional CCBs primarily block L-type calcium channels, while novel CCBs also block N-type and/or T-type channels, leading to organ-protective actions. Consequently, each CCB has unique pharmacokinetic profiles and side effects.
Second, DHP-CCBs have a more potent BP-lowering effect than other classes of antihypertensive drugs. They exhibit similar BP-lowering effects within their class and maintain a relatively constant BP-lowering effect throughout the day.
Third, systolic BP variability was found to be lower in patients receiving amlodipine than in those receiving atenolol or lisinopril. This finding partly explains the reduced risk of vascular events in patients treated with amlodipine.
Fourth, CCBs have protective effects against HMOD, including LVH and increased arterial stiffness. Additionally, CCBs suppress atherosclerosis and coronary vasospasm.
Fifth, L/N-CCBs and L/T-type CCBs, when combined with RAS blockers, result in decreased proteinuria and improved kidney function compared to standard L-type CCBs, despite no additional BP-lowering effect.
Sixth, large-scale trials have shown that DHP-CCBs can effectively reduce CVD events in patients with ISH, the elderly, and high-risk patients. Furthermore, these agents, when combined with RAS blockers, can effectively reduce CVD events.
Seventh, CCBs are indicated for conditions including hypertension in the elderly, ISH, angina pectoris, and coronary vasospasm. Non-DHP-CCBs are contraindicated in any high-grade SA or AV block, HFrEF, bradycardia (e.g., heart rate <60 bpm), and when comedications are susceptible to significant drug interactions mediated by P-gp or CYP3A4. DHP-CCBs should be used with caution in patients with tachyarrhythmia, HFrEF (class III or IV), and preexisting severe edema, while non-DHP-CCBs should be used with caution in patients with constipation.
Eighth, CCB-induced edema is a common side effect and is more common at higher doses. Reducing the dose, switching from DHP to non-DHP-CCBs or lipophilic CCBs, or using combined therapy with a RAS blocker instead of DHP-CCB monotherapy, can lower the risk of peripheral edema development.
Accordingly, CCBs are indicated for a variety of conditions, including hypertension in the elderly, ISH, angina pectoris, and coronary vasospasm. However, it is important to note that each CCB has unique pharmacokinetics and side effects. This necessitates careful consideration when making clinical decisions.
Notes
Ethics statements
Not applicable.
Conflicts of interest
The author has no conflicts of interest to declare.
Funding
None.

