Changes in Target Achievement Rates after Statin Prescription Changes at a Single University Hospital
Article information
Abstract
Background
We investigated the changes in low-density lipoprotein cholesterol (LDL-C) target achievement rates (<70 and <100 mg/dL) when the prescription changed from various statins to Lipilou®, a generic formulation of atorvastatin.
Methods
This was a retrospective cohort study of patients who had been prescribed Lipilou® for more than 3 months at Seoul National University Hospital from 2012 to 2018. For patients who were treated with a previous statin before the prescription of Lipilou®, changes in target achievement rates of LDL-C less than 70 and less than 100 mg/dL were confirmed 3–6 months after the prescription of Lipilou®.
Results
Among the 683 enrolled patients, when their prescription was changed to Lipilou®, the target achievement rate of LDL-C significantly increased for LDL-C less than 70 mg/dL (from 22.1% to 66.2%, p<0.001) and less than 100 mg/dL (from 26.8% to 75.3%, p<0.001). In particular, when a moderate-low potency statin was changed to Lipilou® (10 mg), the target achievement rates for LDL-C less than 70 mg/dL (from 28.9% to 66.7%, p<0.001) and less than 100 mg/dL (from 42.2% to 86.7%, p<0.001) significantly increased. The change from a moderate-high potency statin to Lipilou® (20 mg) showed an increased target achievement rates for LDL-C <70 mg/dL (from 33.3% to 80.0%, p=0.008) and 100 mg/dL (from 40.0% to 73.3%, p<0.025).
Conclusions
We cannot simply conclude that Lipilou® is superior to other statins. However, when the target LDL-C was not reached with previous statin treatments, a high target achievement rate could be achieved by changing the prescription to Lipilou®. Physicians should always consider aggressive statin prescription changes for high target achievement rates.
INTRODUCTION
Cardiovascular disease (CVD) is one of the leading causes of death worldwide and has resulted in 17 million deaths in 2008, accounting for 30% of all deaths.1) With this current trend, it is estimated that the number of deaths from CVD will rise from 17.5 million in 2012 to 22.2 million by 2030.2)3) Dyslipidemia is a major risk factor for the development of CVD,4)5) and has been regarded as one of the reasons for the steady increase in CVD in Korea.6) Low-density lipoprotein cholesterol (LDL-C) is the primary target in the management of dyslipidemia,7) and lipid-lowering therapies for secondary prevention of CVD have been reported to reduce the risk of recurrent cardiovascular events, morbidity, and disability.1)5)
Statins, also known as 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, are recommended as the primary treatment for dyslipidemia8) and are the most effective drugs to prevent CVD.9)10) Various guidelines recommend that the LDL-C target value be set lower depending on the risk factors and presence of accompanying diseases; however, even with the 2016 European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) guidelines,11) 2018 American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology (ACE) guidelines,12) and 2019 ESC guidelines,13) the achievement rates of LDL-C target levels are still low. In particular, the target achievement rate of LDL-C is too low in Asians; approximately 50% of Asian patients with dyslipidemia and 70% of high-risk patients with CVD do not achieve their target LDL-C levels.14) Despite the need to actively attempt for statin changes in case the fall of LDL-C control, it is not applied in clinical practice.15)
Currently, atorvastatin is prescribed for most treatments of dyslipidemia in Korea.16)17) Since the expiration of the original patent for atorvastatin in 2008, many generic formulations of atorvastatin have been released to date, and these formulations are not inferior in terms of their effectiveness or tolerability compared to the original atorvastatin. The purpose of this study was to evaluate the efficacy and safety of Lipilou® (Chong Kun Dang Pharmacy Corp., Seoul, Korea), a generic formulation of atorvastatin. This study is an electronic medical record (EMR)-based retrospective cohort study to evaluate the changes in LDL-C target achievement rates when various types of statins or the same type of atorvastatin are changed to Lipilou®.
METHODS
Study population and design
The subjects consisted of patients who have been prescribed the generic atorvastatin drug, Lipilou® (10, 20, and 40 mg), at Seoul National University Hospital from March 1, 2012 to December 31, 2018. Among patients over 18 years old, those who had been prescribed the drug for more than 3 months after visiting the hospital at least twice and had recorded LDL-C measurements were enrolled. Patients under the age of 18 years or who were prescribed the drug for less than 3 months were excluded from the study.
Study design
The date of the first prescription was defined as the index date (baseline) of the subjects, and the follow-up 3–6 months after the index date was defined as visit 1. Height, weight, and blood test data that were measured within the index date and visit 1 were extracted, including blood urea nitrogen (BUN), creatinine (Cr), aspartate aminotransferase (AST), alanine aminotransferase (ALT), total cholesterol (TC), triglyceride, LDL-C, and high-density lipoprotein cholesterol. If there were more than two blood tests within this period, the measurement taken closer to 90 days from the index date was selected.
Other types of statins that were administered before the index date were also determined. After shifting treatment from other types of statins to Lipilou®, the therapeutic thresholds of LDL-C less than 70 mg/dL and less than 100 mg/dL were identified. Previous statin treatments were divided into four types according to their intensity: high potency such as rosuvastatin (20 mg) and atorvastatin (40 mg); moderate-high potency such as pitavastatin (4 mg), simvastatin (40 mg), rovastatin (10 mg), and atorvastatin (20 mg); moderate-low potency such as fluvastatin (80 mg), pitavastatin (2 mg), pravastatin (40 mg), simvastatin (20 mg), rovastatin (5 mg), and atorvastatin (10 mg); and low potency such as fluvastatin (40 mg) and pravastatin (10 or 20 mg).
Protection of privacy
This study is an EMR-based retrospective cohort study, wherein all data was extracted after anonymization and stored as an encrypted file. This study was approved by the institutional review board of the Seoul National University Hospital Institutional Review Board. Owing to the anonymity of the data and the retrospective nature of the study, informed consent was not required.
Statistical analysis
Data was expressed as means ± standard deviations for continuous variables, and numbers including percentages for categorical variables. The comparison of LDL-C as a normal range between the baseline time up to 3–6 months was performed using McNemar's test by considering the intensity and dosage of the statin. The association between statin prescription and lipid profiles or clinical variables was assessed using a paired t-test. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA), and p-values less 0.05 were considered statistically significant.
RESULTS
A total of 3,991 patients who have been prescribed generic atorvastatin at least once at the Seoul National University Hospital were identified. Among them, 714 patients with a short-term (less than 60 days) prescriptions, 1,449 patients without laboratory test results, 619 patients without baseline LDL-C results, and 526 patients without follow-up LDL-C results were excluded. After these exclusions, 683 patients were included in this study (Figure 1).
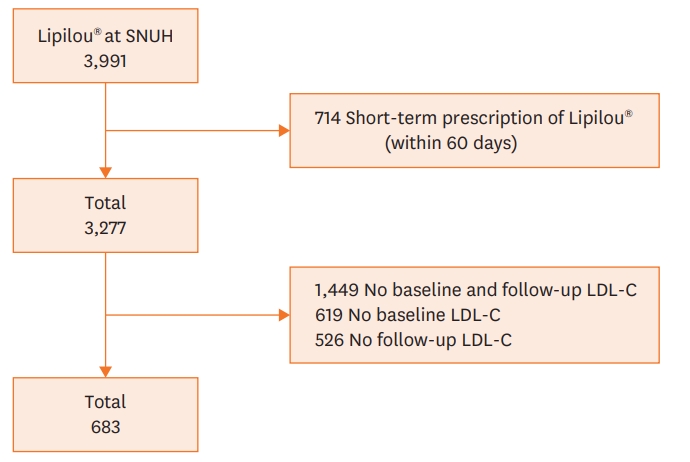
Flow chart describing the process of patient enrollment. Among the 3,991 patients who had a prescription history of Lipilou®, 683 patients who met the criteria have been enrolled.
LDL-C = low density lipoprotein cholesterol; SNUH = Seoul National University Hospital.
The patients included in this study had an average age of 63.4±11.3 years and an average BMI of 24.6±3.4 kg/m2 (Table 1). Lipilou® at a dose of 10 mg was most frequently prescribed (71.7%, 490/683 patients), followed by dose of 20 mg (27.2%, 186/683 patients) and 40 mg (1.0%, 7/683 patients). When examining 96 statins used before the prescription, the most frequent change was from a moderate-low potency statin to Lipilou® (69.8%, 67/96 patients), followed by a moderately potent statin (24.0%, 23/96 patients), a high potency statin (5.2%, 5/96 patients), and a low potency statin (1.0%, 1/96 patients). For 587 patients (85.9%, 587/683 patients), the previous statin prescription could not be confirmed.
When the statin prescription was changed to Lipilou®, the achievement of therapeutic thresholds of LDL-C less than 70 mg/dL (from 22.1% to 66.2%, p<0.001) and less than 100 mg/dL (from 26.8% to 75.3%, p<0.001) increased significantly (Table 2). After a prescription change from a moderate-low potency statin to Lipilou®, the achievement of therapeutic thresholds of LDL-C were found to increased significantly. In particular, in an equivalent potency group, wherein a moderate-low potency statin was changed to Lipilou® (10 mg), the achievement rates for LDL-C less than 70 mg/dL (from 28.9% to 66.7%, p<0.001) and less than 100 mg/dL (from 42.2% to 86.7%, p<0.001) were significantly increased. On the other hand, the change from a moderately high potency statin to Lipilou® (20 mg) resulted in increased achievement rates of therapeutic thresholds of LDL-C less than 70 mg/dL (from 33.3% to 80.0%, p=0.008) and 100 mg/dL (from 40.0% to 73.3%, p=0.025). When the potency was lowered from a moderate to high potency statin to Lipilou® (20 mg), the achievement rates for therapeutic thresholds of LDL-C less than 70 mg (from 50.0% to 75.0%, p=0.317) and less than 100 mg (from 50.0% to 100.0%) increased, but were not statistically significant.
Table 3 shows the results of prescription changes from the same atorvastatin family to Lipilou®. All 66 cases where the atorvastatin prescription was changed to Lipilou® showed significantly increased achievements of therapeutic thresholds of LDL-C less than 70 mg/dL (from 33.3% to 77.3%, p<0.001) and less than 100 mg/dL (from 42.4% to 80.3%, p<0.001) compared to baseline (Table 3). When a moderate-low potency statin prescription was changed to Lipilou® (10 mg), the achievement rates of therapeutic thresholds of LDL-C less than 70 mg/dL (from 35.3% to 73.5%, p<0.001) and less than 100 mg/dL (from 47.1% to 88.2%, p<0.001) significantly increased. When a moderate-high potency statin prescription was changed to Lipilou® (20 mg), the achievement rates for LDL-C therapeutic thresholds less than 70 mg/dL (from 33.3% to 100.0%) and less than 100 mg/dL (from 44.4% to 77.8%, p=0.025) increased. When the prescription was changed from a moderate-to-high potency statin to Lipilou® (20 mg), there were increased achievement rates for therapeutic thresholds of LDL-C less than 70 mg (from 50.0% to 75.0%, p=0.317) and less than 100 mg (from 50.0% to 100.0%), but no meaningful trends were found.
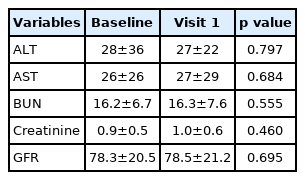
We also assessed changes in liver and kidney blood test results after the prescription (Table 4), wherein there were no significant differences in the values at baseline compared to 3 months after the prescription change. We found that that 0.2% (1/594 patients) of patients had their AST increased more than 3 times the normal upper limit, while 0.3% (2/678 patients) of patients had their ALT increased more than 3 times the normal upper limit.
DISCUSSION
Dyslipidemia is a major factor that contributes to CVD in Korea, and a reduction of LDL-C by 1.0 mmol/L has been reported to decrease the number of cases of CVD by 20%.18)19) Nevertheless, the perception and treatment rate of dyslipidemia is low in Asia, with untreated and uncontrolled high-risk patients accounting for 60% and up to 30% of all cases, respectively.14)20)21) In Korea, only 2 of 5 patients have been reported to reach their TC target levels.22) For this reason, medical staff should check the achievement of LDL-C target levels after statin prescription, and changes in either the dosage or type of statin need to be considered for cases that do not reach these target levels.
In this study, data of 683 patients with generic atorvastatin and Lipilou® prescriptions were extracted from 7 years of EMRs at Seoul National University. In patients who have been taking statins and switched their prescription to generic atorvastatin, the target achievement rate of LDL-C increased significantly. In particular, when the prescription was changed from a moderate-low statin to 10 mg of generic atorvastatin or from a moderate-high potency statin to 20 mg of generic atorvastatin, where the drop effect of LDL-C is known to be the same, the achievement rate was significantly increased for both LDL-C targets of less than 70 mg/dL and less than 100 mg/dL. In fact, it is difficult to evaluate the increased target achievement as a single effect of this generic drug because the statin change itself can affect patient compliance. However, if the LDL-C value does not reach the target level, simply changing to the prescription to a generic formulation of atorvastatin can help in significantly reducing LDL-C levels.
In the 2016 ESC/EAS,11) 2018 AACE/ACE,12) and 2019 ESC guidelines,13) the target LDL-C value was set lower. Despite the established guidelines for the target values of LDL-C, treatment gaps that do not reach the target level exist in actual clinical settings, and clinicians should actively focus on reducing these. Among the various ways to achieve this, active statin change is a primary consideration when the goal of the LDL-C is not reached.
Similar results were obtained when the prescription was changed from other generic atorvastatin agents. Even in cases where a statin of equivalent potency was changed to Lipilou®, the target achievement rate of LDL-C tended to increase. In the KoLipilou study of CVD patients in Korea, there was no difference in LDL-C levels between the two groups who were prescribed original atorvastatin and Lipilou®, and there was no significant difference in the incidence of side effects.23) In the LAMP study, a real-world observational study of Lipilou®, the achieved therapeutic thresholds of LDL-C were high (69%–80%), especially in patients taking a moderate-potency statin.24) This demonstrates that the generic formulation of atorvastatin is objectively efficacious and safe, thereby providing supporting evidence for active statin prescription changes.
After the prescription changes to Lipilou®, other laboratory results such as ALT, AST, BUN, creatine, and eGFR levels did not show any statistically significant changes. In particular, the increase in ALT to 3 times more than a normal value was only found in 0.3% of patients. If the AST or ALT level was more than 3 times higher than that of upper normal limit after the statin prescription, it can be considered that the incidence is considerably lower than that known by 0.5–2.0%.25) Even though this study has a short follow-up period of 3–6 months, our results are clinically meaningful because in previous studies, the increase in AST or ALT level due to statins mainly occurs in the first 3 months of treatment.
Since this study is an EMR-based retrospective cohort study of a single institution, it has some limitations.26)27) First, due to the nature of a retrospective cohort study, a large amount of data was missing. In fact, this study was conducted in about 20% of patients who were prescribed Lipilou®. Moreover, it is difficult to ascertain the case history of statin prescriptions from another hospital. If the prescription was not properly written in the medical record, it can be difficult to confirm even if a direct chart review by medical staff is conducted. Because of this, cases were classified as “unknown” if it was uncertain whether other statins were prescribed prior to the Lipilou® prescription. Therefore, the relatively small presence of former statins is also a limitation. However, this study provides real-world evidence based on EMR data; this study reported the achievement rates of therapeutic thresholds of LDL-C in patients taking generic atorvastatin in real-world clinical practice.
The results of this study showed that this generic agent was not inferior to other types of statins or original statins in terms of efficacy and stability. Moreover, if the LDL-C level cannot be reached with the existing statins, an increased achievement of target rate can be expected only by actively switching to Lipilou®. Aggressive prescription changes may also be worth considering. However, it is difficult to conclude from this study whether the specific agent is superior to other types of statins. In order to objectively support the results of this study, prospective large-scale studies will be needed, and we hope that this study can be a basis for these future studies.
Notes
Funding
The current study was financially supported by the Chong Kun Dang Pharm (Seoul, Korea), who had no role in data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors have no financial conflicts of interest.
Author Contributions
Conceptualization: Choe S; Data curation: Kim HS, Kim JH; Formal analysis: Kim HS, Kim JH; Methodology: Choe S; Supervision: Kim HS, Kim JH; Writing - original draft: Choe S; Writing - review & editing: Shinn J.