Preterm Labor and Later Maternal Cardiovascular Disease in General Population: Doubtful Relationship with Atherosclerosis
Article information
Abstract
Background
The maternal cardiovascular system experiences an enormous challenge during pregnancy. A history of preterm labor suggestive of dysfunctional pregnancy might be associated with the maternal later life chronic cardiovascular disease (CVD). We evaluated the association between preterm labor and the late development of maternal atherosclerotic CVD using the national database of general population.
Methods
Data for 5,226 postmenopausal women aged ≥50 years were analyzed from the Korean National Health and Nutrition Examination Survey V, which had conducted from 2010 to 2012.
Results
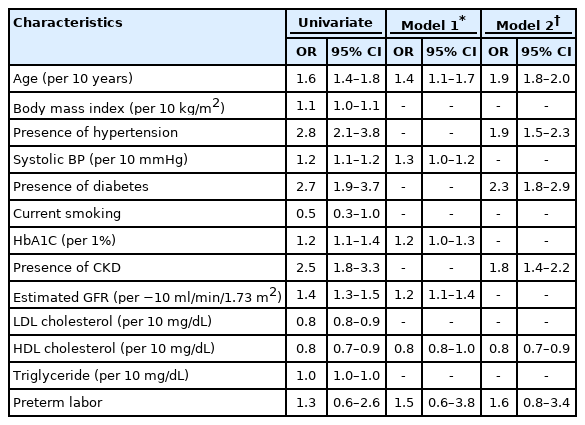
The numbers of preterm labor and CVD (stroke, myocardial infarction, or angina pectoris) were 151 (3.0±0.3%) and 367 (6.6±0.4%), respectively. In a multivariate analysis, CVD was independently associated with age (odds ratio [OR], 1.9; 95% confidence interval [CI], 1.8–2.0), the presence of hypertension (OR, 1.9; 95% CI,1.5–2.3), diabetes (OR, 2.3; 95% CI, 1.8–2.9), chronic kidney disease (OR, 1.8; 95% CI, 1.4–2.2) and low high-density lipoprotein cholesterol concentration (OR, 0.8; 95% CI, 0.7–0.9) were independently associated with CVD. A history of preterm labor was not associated with CVD (OR, 1.5; 95% CI, 0.6–3.8).
Conclusions
There was no significant association between preterm labor and atherosclerotic CVD in general population. A history of preterm labor is not likely to be a risk factor for later CVD in women.
INTRODUCTION
Research on cardiovascular disease (CVD) has focused mostly on male patients, and the results have been assumed to be similar in females. However, recently, it was recognized that CVD had unique characteristics in females, and efforts have been actively directed toward finding women-specific risk factors for CVD.1)2) The maternal cardiovascular system sustains an enormous challenge during pregnancy. Pregnancy serves as a natural stress test for unveiling subtle abnormalities and provides an opportunity to screen for subclinical CVD.3)4) It is known that women that experience pregnancy complicated by hypertension or diabetes are likely to be affected by chronic hypertension or diabetes in later life.1)5-7) In a similar vein, other common pregnancy events, including preterm labor or delivery of baby with low birth weight were associated with the later development of maternal chronic disease.6)8-18) We examined that National database to evaluate the prevalence of preterm labor and later development of maternal CVD.
METHODS
Data source and population
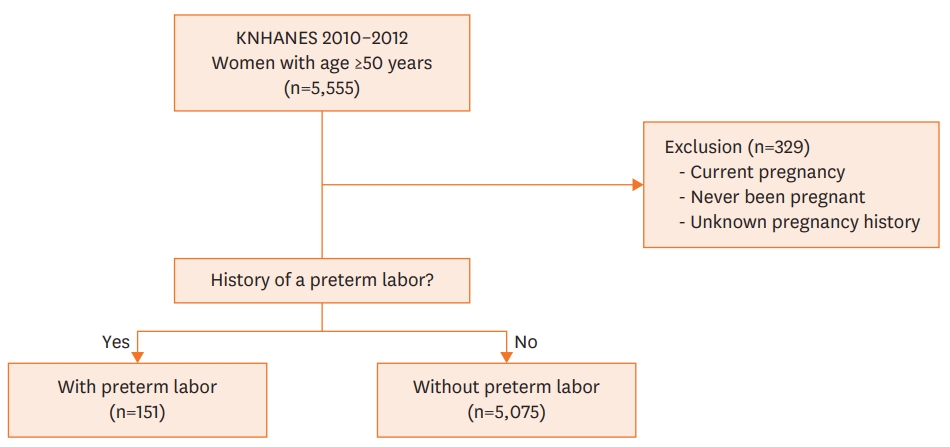
The present cross-sectional study was based on the dataset from the Korean National Health and Nutrition Examination Survey V (KNHANES V, 2010–2012). Briefly, the KNHANES project has conducted surveys periodically from 1998 to the present to obtain nationally representative data about the health and nutritional status of the general Korean population. The study implements a stratified multistage clustered probability sampling design to collect data that represents the entire population of non-institutionalized Korean citizens.19) These data are available on a public website (http://knhanes.cdc.go.kr).20) In the present study, data for post-menopausal women, aged ≥50 years were selected from a total of 5,555 subjects in the KNHANES V dataset. Women that had never been pregnant or was been pregnant at the time of survey were excluded (n=329). The final population included 5,226 subjects (Figure 1). When we applied pre-specified statistical weights, our study population was estimated to represent 7,525,649 civilians of post-menopausal women, aged ≥50 years.
Definitions
Preterm labor was defined as a history of any preterm labor. CVD was defined as a history of stroke, myocardial infarction, or angina pectoris, diagnosed by physician. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or current use of anti-hypertensive medications. Diabetes was defined as current use of hypoglycemic medication or a history of diabetes diagnosed by a physician. Chronic kidney disease (CKD) was defined as estimated glomerular filtration rate (GFR) <60 mL/min/1.73 m2 by the Chronic Kidney Disease Epidemiology Collaboration formula.21)
Measurements
Blood pressure was measured with a mercury sphygmomanometer, with the participant in a sitting position. Measurements were repeated three times at 5-min intervals, and the average of the second and third values was used in this study. Blood samples were drawn after at least 10 hours of overnight fasting. Samples were transferred to a central laboratory for measurement. Total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and triglyceride were measured enzymatically with a Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan). When a LDL cholesterol value was missing in patient data, we imputed the value for subjects with triglyceride concentrations <200 mg/dL as follows: LDL cholesterol=total cholesterol−HDL cholesterol−5/triglyceride.22) Hemoglobin A1C (HbA1C) was measured with high performance liquid chromatography on a HLC-723G7 apparatus (Tosoh, Tokyo, Japan). Creatinine was measured with the Jaffe method on a Hitachi Automatic Analyzer 7600 (Hitachi).
Statistical analysis
All statistical analyses were performed with SAS software, version 9.2 (SAS Institute Inc, Cary, NC, USA). For each analysis, we used pre-specified statistical commands to adjust the complex design of the dataset. Continuous data are expressed as the mean±standard error (SE) evaluated with the SAS analysis tool, “SURVEYMEANS.” The differences between groups were tested with the “SURVEYREG” tool. Frequency data are presented as percentage±SE from the “SURVEYFREQ” feature, based on a Rao-Scott chi-square analysis. Associations between known demographic or laboratory CVD risk factors were estimated with a logistic regression analysis (SAS analysis tool, “SURVEYLOGISTIC”). The final model included age, preterm labor, and parameters that showed significance in a univariate analysis. Two-sided p values <0.05 were considered significant.
Ethics statement
The study protocol was approved by the Institutional Review Board (IRB) of the Korea Center for Disease Control and Prevention (IRB No. 2010-02CON-21-C, 2011-02CON-06-C, 2012-01EXP-01-2C).19)20) All participants in the KNHANES provided written informed consent prior to study initiation.
RESULTS
Profile of the population
Among 5,226 study population, a history of preterm labor was reported in 151 (3.0±0.3%) subjects. Overall CVD was reported in 367 (6.6±0.4%), in which the number of stroke, myocardial infarction and angina pectoris were 142 (2.6±0.3%), 57 (1.0±0.2%) and 196 (3.6±0.3%), respectively. CKD was reported in 652 (12.3±0.6%, Table 1). When patients were grouped by decades of age, the number of women with CVD were 60 (3.2±0.5%) for 50-59; 133 (8.5±0.8%) for 60-69; 143 (10.1±1.0%) for 70-79; and 31 (11.1±2.2%) for 80-89 years of age, respectively (p trend < 0.001). The proportion of women with a history of preterm labor decreased with increasing age. The numbers of women with a history of preterm labor in each decade of age were 65 (3.4±0.6%) for 50-59; 59 (3.6±0.6%) for 60-69; 26 (1.9±0.4%) for 70-79; and 1 (0.1±0.1%) for 80-89 years, respectively (p trend=0.002).
Preterm labor and late maternal CVD
The demographic and laboratory characteristics of women with or without a history of preterm labor are shown in Table 1. Among this group of post-menopausal women, women with a history of preterm labor were younger than women without preterm labor (60±0.9 years vs. 63±0.2 years, p=0.001). The rate of angina pectoris, myocardial infarction, stroke, and overall CVD were not different between women with and those without preterm labor histories (Figure 2).
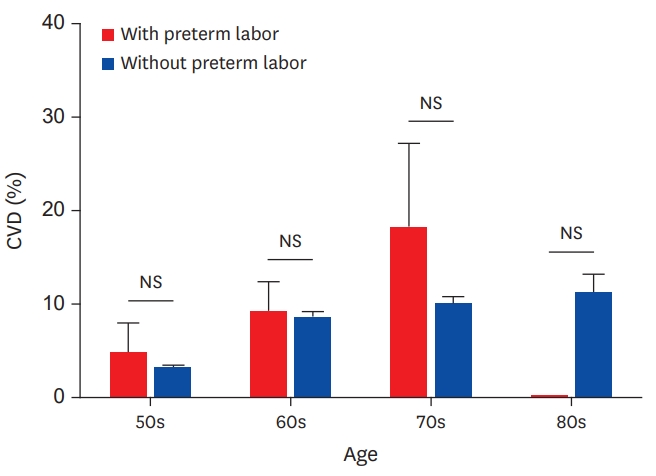
Prevalence of CVD in increasing age groups, with or without a history of preterm labor.
CVD = cardiovascular disease; NS = not significant.
The relationship between baseline characteristics and the prevalence of CVD is shown in Table 2. In a multivariate logistic regression analysis, age (odds ratio [OR], 1.9; 95% confidence interval [CI], 1.8–2.0), the presence of hypertension (OR, 1.9; 95% CI, 1.5–2.3), diabetes (OR, 2.3; 95% CI, 1.8–2.9), CKD (OR, 1.8; 95% CI, 1.4–2.2) and low HDL cholesterol (OR, 0.8; 95% CI, 0.7–0.9) were independently associated with CVD. However, a history of preterm labor was not a significant factor for CVD (OR, 1.5; 95% CI, 0.6–3.8).
Additional analysis
LDL cholesterol and the current smoking status were not associated with CVD in the present study. The mean LDL cholesterol concentrations in age groups of 50–59, 60–69, 70–79, and 80–89 years were 130±1.0, 124±1.0, 122±1.2, and 127±3.0 mg/dL, respectively (p<0.001). Medical treatment for dyslipidemia might affect such results. The number of women taking dyslipidemia medications were 183 (8.7±0.7%), 305 (19.2±1.2%), 182 (14.4±1.2%), and 14 (3.8±1.3%), respectively (p<0.001).
DISCUSSION
The present study showed that a history of preterm labor was not associated with maternal CVD in the general Korean population. The prevalence of CVD including stroke, myocardial infarction and angina were not different between women with and those without a history of preterm labor. The number of subjects with a history of preterm labor decreased disproportionally with age.
Pregnancy provides a natural stress test and a window to future health, there has been concern over the relationship between pregnancy complications and life-long health in women.4) Pregnancy in late age may be dysfunctional and face to many challenges. It is frequently associated with poor obstetric outcomes including preterm labor and various medical problems. Pregnancy-related hypertension, including preeclampsia, is a well-known risk factor for atherosclerotic CVD.1)7) Endothelial dysfunction appears to play a role in inadequate placental circulation and increased systemic vascular resistance in preeclampsia. Endothelial dysfunction and subsequent inflammation might eventually contribute to atherosclerotic ischemia and CVD over the long term.23)
Preterm labor was also known to be associated with a poor maternal outcome.10)14) According to nationwide Swedish data, a low birth weight for gestational age was associated with maternal all-cause and CVD mortality. That result was not confounded by smoking, but the possible role of genetic factors was suggested.12) Another study based on Swedish data showed that preterm labor or severe fetal growth retardation was associated with later maternal hospitalization or death from coronary heart disease, cerebrovascular events, and heart failure.16)
Our results contradicted such previous studies. This disparity could be explained in several ways. First thing is the ethnic differences among study populations. Most previous studies on preterm labor and CVD were conducted in Nordic countries and Scotland.8-16) Few reports from other regions have been published. Second, the heterogeneity CVD definitions among different reports should be considered. Among the wide spectrum of CVDs, a variety of mechanisms may be involved. As the pathophysiology of atherosclerotic and nonatherosclerotic diseases is quite different, the relationship between premature labor and CVD might be different among individual studies. Third, the causes of preterm labor are complex. Non-medical factors including socioeconomic status can be complicated.24) Lastly, the cause, age, and severity of preterm labor can be matter. For example, mothers that had elected preterm labors, in which vaginal or cesarean delivery was induced, due to maternal or fetal status, showed greater associations between APE and ischemic heart disease than mothers that had spontaneous preterm deliveries.15) Also, extremely early pre-term births and severe fetal growth retardation were more highly associated with maternal risk of CVD than less severe cases.15)16) In a Danish cohort, preterm labor and fetal growth retardation were strongly associated with early maternal mortality from cardiovascular and non-cardiovascular causes. However, pregnancy-related hypertension was only associated with CVD.14)
Although literature suggested that preterm labor is associated with poor long-term outcomes, we strongly doubt that atherosclerotic CVD is associated with preterm labor based on the present findings. Conventional cardiovascular risk factors were independently associated with CVD with exception of LDL cholesterol (Table 2), where the high prevalence of statin therapy among older women may play a role. The parameters that represented atherosclerotic risk factors, including the mean systolic and diastolic blood pressures, a history of hypertension or diabetes, the lipid profile, and renal function, were not significantly related to women with premature labor. More research is needed to conclude whether preterm labor should be considered a women-specific cardiovascular risk factor.
The present study had several limitations. First, the study had a cross-sectional design; thus, causal relationships could not be assessed. Second, the possibility of errors in exposure measurements, including recall bias, is present. Lastly, KNHANES is public database for general population and does not include the detailed information about CVD and pregnancy complications.
In conclusion, there was no significant association between preterm labor and atherosclerotic CVD in KNHANES dataset. A history of preterm labor is likely not to be a risk factor for later CVD in women.
Notes
Funding
This work was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI14C1731).
Conflict of Interest
The authors have no financial conflicts of interest.
Author Contributions
Conceptualization: Lee KY; Data curation: Han SW, Lee KY; Formal analysis: Han SW, Nam HS, Lee KY; Funding acquisition: Lee JH, Lee KY; Investigation: Han SW, Kim YJ, Seo WK, Yu S, Nam HS, Yoon SS, Kim SH, Lee JY, Lee JH, Hwang YH, Lee J, Lee KA, Lee KY; Methodology: Kim YJ, Kim SH, Hwang YH, Lee KA, Lee KY; Project administration: Kim YJ, Lee KY; Resources: Lee J, Lee KY; Supervision: Lee KY; Validation: Yu S, Nam HS, Lee KA, Lee KY; Visualization: Yu S, Lee KA; Writing - original draft: Han SW; Writing - review & editing: Lee KY.