ABSTRACT
-
Background
- The relationship between metformin intake and prostate cancer incidence remains unclear. Therefore, we examined the correlation between prostate cancer and metformin use.
-
Methods
- The subjects were diabetes patients aged ≥50 years who had been diagnosed with prostate cancer and had undergone surgery at Seoul St. Mary's Hospital. Groups taking metformin (MET(+) group) and not taking metformin (MET(–) group) were divided and compared.
-
Results
- The mean preoperative prostate-specific antigen (PSA) levels in the MET(–) and MET(+) groups were 10.7±11.9 and 8.0±5.6 ng/mL, respectively, with no statistically significant difference between the two groups (P=0.387). The average prostate volume of the MET(–) group was 82.4±98.0 mL, and the average prostate volume of the MET(+) group was 55.4±20.1 mL, but there was no statistically significant difference between the two groups (P=0.226). The mean PSA velocity also did not show a significant difference between the two groups (0.025±0.102 ng/mL vs. 0.005±0.012 ng/mL, P=0.221).
-
Conclusions
- We did not identify a significant positive correlation between metformin and prostate cancer. However, preoperational PSA and PSA velocity tended to be lower in the MET(+) group. A sophisticated prospective study with a large sample size should be planned.
-
Keywords: Metformin; Prostate neoplasms; Diabetes mellitus
INTRODUCTION
- Most studies have reported a higher rate of cancer incidence in diabetes patients than in people without diabetes [1–3]. The factors affecting the cancer incidence rate in diabetes patients are obesity, decreased physical activity, smoking, and drinking. Metformin (1,1-dimethylbiguanide hydrochloride), a medication for diabetes, has gained prominence due to its significant role in cancer prevention, in addition to its blood glucose-lowering effects [4,5]. Theoretically, metformin not only activates AMP-activated protein kinase (AMPK)—which inhibits the growth of cancer cells [6]—but also deters cancer through a mechanism that reduces insulin-like growth factor-1 (IGF-1) levels [7], which typically promote cancer development. Consequently, metformin has attracted interest as a novel approach to cancer treatment, necessitating further comprehensive clinical research. Metformin administration is known to have a preventive effect on cancer, and this effect has also been observed in individuals without diabetes.
- Recent studies have reported conflicting findings regarding the effects of metformin on patients with prostate cancer. Most studies have reported that metformin lowers the mortality rate of prostate cancer patients and decreases the risk of other causes of death [8–10]. However, some studies have reported no effect on all-cause mortality [11,12]. A study emphasized that the cumulative dosage of metformin had a greater effect on cancer-related outcomes than any single dose of metformin [5]. Specifically, the risk of cancer decreased as the total amount of metformin dispensed increased.
- In summary, we expect varying results regarding the development of prostate cancer based on different metformin dosages. However, there have been no studies to date on the optimal dosage of metformin for treating prostate cancer. Consequently, the objective of this study was to investigate the association between the intake of metformin and the incidence of prostate cancer. Our goal was to determine whether metformin use is associated with the occurrence of prostate cancer.
METHODS
- Ethics statements
- This study was approved by the Institutional Review Board of The Catholic University of Korea (No. KC19RNSI0810). As the data were collected retrospectively and anonymized, individual informed consent from the patients was not required. The study adhered to the tenets of the Declaration of Helsinki.
- Study population
- This study included patients aged 50 years and above, diagnosed with diabetes and prostate cancer, who underwent surgery at Seoul St. Mary’s Hospital, The Catholic University of Korea (Seoul, Korea) between 2006 and 2012. We excluded patients diagnosed with prostate cancer who did not undergo surgery. The diagnostic criteria for prostate cancer were established according to the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) code C61. We defined patients as those diagnosed with diabetes and were on antidiabetic medication. The diagnosis codes for diabetes were determined based on ICD-10 classifications E11–E14. We excluded patients with type 1 diabetes mellitus or those on insulin therapy.
- Study design
- This study investigated whether the patients included had been administered metformin and, if so, the total dosage. Patients who received metformin were categorized into the MET(+) group, while those who did not were designated as the MET(–) group. Personal body information, including sex, age, height, and weight, was extracted for comparisons between the groups, and the body mass index (BMI) was calculated using height and weight. Additionally, known prognostic factors for prostate cancer—such as preoperative prostate-specific antigen (PSA) level, prostate volume, and calculated PSA velocity—were also extracted.
- Privacy protection
- This study was conducted by retrospectively collecting data from the electronic medical records of Seoul St. Mary’s Hospital, and thus entailed no possibility of physical or mental harm to patients. All data were anonymized, stored in encrypted files, and encrypted on the principal researcher’s computer. Without additional information, the individuals corresponding to the data could not be identified.
- Statistical analysis
- Descriptive statistics were presented as mean ± standard deviation or percentage of the total participants. For continuous variables, we tested whether the means or medians of the two groups were equivalent, as follows. When variables were normally distributed for both groups, the t-test was performed. If the test for homogeneity of variance was not rejected, the t-test was performed, assuming equal variances. When variables did not follow a normal distribution, the Wilcoxon rank-sum test was performed. For categorical variables, the chi-square test of homogeneity was performed to test whether the proportions of categories in the two groups were equivalent. For the correlation analysis, Kendall test was performed. The analyses were performed using R ver. 4.2.2 (R Foundation for Statistical Computing). A P-value of <0.05 was considered to indicate statistical significance.
RESULTS
- Between 2006 and 2012, 858 prostate cancer patients aged 50 years or older were diagnosed at Seoul St. Mary’s Hospital (Fig. 1). Of these, 446 patients without diabetes were excluded. Among them, 247 who had been externally diagnosed with diabetes and for whose existing antidiabetic drugs could not be easily identified were excluded. Sixty-five patients were finally included in this study, excluding 100 diagnosed with prostate cancer who had not undergone surgery. Of those 65 patients, 31 were taking metformin, whereas 34 were not.
- Differences in baseline characteristics according to the presence or absence of metformin intake
- The average age was 67.3±6.1 years in the MET(–) group and 62.5±13.1 years in the MET(+) group. There was no significant age difference between the two groups (P=0.818) (Table 1). In the MET(–) group, five of 34 patients (14.7%) were between 50 and 59 years old, 14 patients (41.2%) were between 60 and 69 years old, and 15 patients (44.1%) were 70 years old or older. In the MET(+) group, five of 31 patients (16.1%) were between 50 and 59 years old, 13 patients (41.9%) were between 60 and 69 years old, and 13 patients (41.9%) were 70 years old or older. No significant difference in the distribution of these age groups was noted between the two groups (P=0.980). There was also no significant difference in height (166.8±5.7 cm vs. 167.1±6.1 cm, P=0.854), weight (68.0±7.1 kg vs. 70.3±12.4 kg, P=0.524), or BMI (25.1±3.6 kg/m2 vs. 24.4±1.93.6 kg/m2, P=0.489) between the two groups. The total dosage of metformin in the MET(+) group was 1,269,562±1,530,541 mg.
- Differences in prostate variables according to the presence or absence of metformin intake
- The mean preoperative PSA levels in the MET(–) and MET(+) groups were 10.7±11.9 and 8.0±5.6 ng/mL, respectively, with no significant difference between the groups (P=0.387). (Fig. 2) The average prostate volume in the MET(–) and MET(+) groups were 82.4±98.0 and 55.4±20.1 mL, respectively, with no statistically significant difference between the two groups (P=0.226). The mean PSA velocity also did not differ significantly between the groups (0.025±0.102 ng/mL vs. 0.005 ± 0.012 ng/mL, P=0.221).
- Correlation analysis of variables in the MET(+) group
- In the MET(+) group, no significant correlation was observed between the total dose of metformin and preoperative PSA level (P=0.985), between the total dose of metformin and prostate volume (P=0.691), or between the total dose of metformin and prostate velocity (P=0.496).
DISCUSSION
- Globally, the number of patients with diabetes has surged in recent decades [13]. Numerous epidemiological studies have established that diabetes elevates the risk of various cancers, including pancreatic, breast, endometrial, and bladder cancers [14]. The mechanisms through which diabetes heightens the incidence rates of these cancers encompass cell proliferation due to chronic inflammation and diabetic acidosis, which is worsened by hyperglycemia. Consequently, it is theoretically advisable for individuals to maintain adequate blood glucose control to mitigate the risk of cancer. In the same vein, diabetes medications can potentially reduce these risks. In contrast to other cancers, the relationship between diabetes and the risk of prostate cancer has been extensively researched. Numerous studies have found that patients diagnosed with diabetes are more susceptible to prostate cancer. However, as the duration of a diabetes diagnosis increases [15], the incidence rate of prostate cancer has been observed to decline [16]. In essence, a longer duration of diabetes is associated with a reduced incidence of prostate cancer. While numerous hypotheses have been proposed to explain this phenomenon, the most plausible explanation is the reduced insulin dosage. This that as the duration of diabetes extends, the concentration of insulin decreases in insulin is associated with relatively low testosterone levels the risk of prostate cancer [17].
- According to the literature, all oral hypoglycemic agents that reduce blood glucose levels also decrease the incidence rate of prostate cancer. Specifically, as the dosage and duration of administration increased, there was a suppression of cancer progression. As the duration of treatment with oral hypoglycemic agents and other medications increases, the risk of prostate cancer decreases in patients with diabetes. One study showed that male participants who were administered antidiabetic drugs such as metformin, sulfonylurea, and insulin had a 16% reduced risk of prostate cancer. This study also identified factors that influenced the duration and dosage of diabetic medications [18]. Male patients who had been diagnosed with diabetes within the past three years exhibited a slightly higher risk of prostate cancer compared to those without diabetes. However, some patients who had been living with diabetes for more than four years demonstrated a one-third reduction in the risk of prostate cancer [19]. Interestingly, while the early stages of diabetes increase the risk of prostate cancer, the risk decreases over time. There appeared to be no significant difference in the ability of various oral hypoglycemic agents to reduce the risk of cancer, but metformin was found to inhibit the proliferation (or division) of prostate cancer cells [20]. Metformin is an oral hypoglycemic agent that is commonly used as the first-line treatment for patients with type 2 diabetes. This medication has recently gained renewed attention due to its effectiveness in cancer treatment. While further research is required, many existing studies suggest that metformin reduces both the incidence of cancer and mortality rates following a prostate cancer diagnosis. Despite the promising results associated with metformin, this study was unable to confirm its preventive effect on prostate cancer due to the small sample size.
- Metformin is known for its ability to reduce mortality rates by inhibiting the proliferation of prostate cancer cells. A study involving 3,837 individuals diagnosed with both diabetes and prostate cancer found that the administration of metformin reduced the mortality rate from prostate cancer by 24% every 6 months. As the duration of metformin administration lengthened, the mortality rate from prostate cancer and other causes also declined. Metformin was found to increase the survival rate of prostate cancer patients [10] and reduce the mortality rate from other causes. Other antidiabetic drugs, with the exception of metformin, did not demonstrate a significant reduction in the mortality rate. Finnish research [21] conducted over seven years with 50,000 subjects reported that as the duration of diabetic medication use increased, the risk of prostate cancer decreased also found that the growth of cancer cells was inhibited in proportion to the duration of administration in patients who had already been diagnosed with prostate cancer. Therefore, metformin prevents the risk and exacerbation progression of prostate cancer in proportion to the duration of medication used to lower blood glucose levels in patients with diabetes [22]. Another study divided 13,409 patients who had type 2 diabetes with prostate cancer to evaluate the effects of metformin. The results indicated that while metformin did not increase the overall risk of prostate cancer, it did increase the risk of low-grade prostate cancer by 14%, and conversely, it decreased the risk of high-grade prostate cancer by 25% [12].
- Numerous studies have indicated that metformin not only reduces the incidence of cancer but also decreases the mortality rate associated with it [23]. Specifically, metformin has been demonstrated to inhibit the proliferation of prostate cancer cells. While there is no definitive mechanism explaining how metformin reduces the risk of prostate cancer, several hypotheses have been put forward. The most credible of these suggests that an increase in morbidity is associated with a decrease in insulin concentration [24], leading to a subsequent reduction in IGF-1. This process leads to the suppression of the human epidermal growth factor receptor-2, thereby lowering the incidence of prostate cancer [25]. Metformin also activates AMPK, which inhibits cancer cells [5] and displays antitumor effects. As testosterone levels decrease with prolonged diabetes, the incidence of prostate cancer testosterone contributes to the hypertrophy of prostate tissue and accelerates the rapid progression of cancer [26].
- Although metformin has shown various positive effects on prostate cancer, we were unable to establish a clear correlation in this study. However, due to the limitations of a small sample size and retrospective study design, it is challenging to definitively state that there is no association between metformin and prostate cancer [27,28]. To address these limitations, a prospective study with a larger sample size is necessary. Furthermore, given that blood glucose control can potentially influence tumor growth, a multivariable analysis that includes factors such as blood glucose, hemoglobin A1c, and other medications used in conjunction with metformin should be conducted to reduce biases. Future studies should aim for a larger sample size and a more rigorous study design.
- As research into the anticancer effects of metformin, a primary diabetes medication, continues to expand, there is increasing interest in its potential application for cancer patients. Most studies examining the efficacy of metformin have focused on patients with both diabetes and prostate cancer. However, there have been no studies that confirm the same effect in patients diagnosed solely with prostate cancer. Furthermore, the specific role and mechanism of action of metformin in prostate cancer have yet to be definitively established. While this study did not find a positive correlation between metformin and prostate cancer, a randomized controlled trial with a larger sample size is necessary to further explore the potential of metformin.
ARTICLE INFORMATION
-
Ethics statements
This study was approved by the Institutional Review Board of The Catholic University of Korea (No. KC19RNSI0810). As the data were collected retrospectively and anonymized, individual informed consent from the patients was not required. The study adhered to the tenets of the Declaration of Helsinki.
-
Conflicts of interest
The authors have no conflicts of interest to declare.
-
Funding
This work was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science and ICT of Korea (No. NRF-2019M3E5D3073104).
-
Author contributions
Conceptualization: RK, MS, HSK; Data curation: all authors; Formal analysis: RK, MS, HSK; Funding acquisition: HSK; Investigation: HSK; Methodology: HSK; Project administration: HSK; Resources: HSK; Software: HSK; Supervision: HSK; Validation: HSK; Visualization: RK, MS; Writing–original draft: RK, MS; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Fig. 1.Inclusion and exclusion of patients. MET(–), patients not taking metformin; MET(+), patients taking metformin.
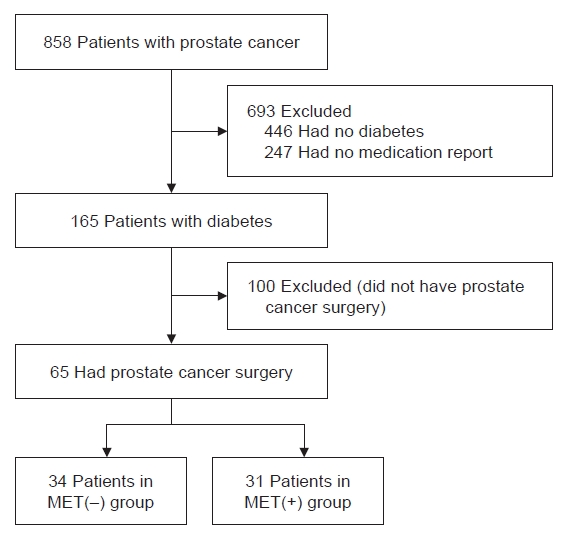
Fig. 2.Differences in prostate variables according to the presence or absence of metformin intake. (A) The mean preoperative prostate-specific antigen (PSA) levels. (B) The average prostate volume. (C) The mean PSA velocity. MET(–), patients not taking metformin; MET(+), patients taking metformin.
Table 1.Baseline characteristics
|
Characteristic |
MET(–) group (n=34) |
MET(+) group (n=31) |
P-value |
|
Age (yr) |
67.3±6.1 |
65.2±13.1 |
0.818 |
|
50–59 |
5 (14.7) |
5 (16.1) |
0.980 |
|
60–69 |
14 (41.2) |
13 (41.9) |
|
|
≥70 |
15 (44.1) |
13 (41.9) |
|
|
Height (cm) |
166.8±5.7 |
167.1±6.1 |
0.854 |
|
Weight (kg) |
68.0±7.1 |
70.3±12.4 |
0.524 |
|
Body mass index (kg/m2) |
24.4±1.9 |
25.1±3.6 |
0.489 |
|
Metformin dose (mg) |
0 |
1,269,562±1,530,541 |
|
REFERENCES
- 1. Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA 2005;293:194–202.ArticlePubMed
- 2. Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. Diabetes Care 2010;33:1674–85.ArticlePubMedPMC
- 3. Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer 2009;16:1103–23.ArticlePubMed
- 4. Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451–61.ArticlePubMedPDF
- 5. Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005;330:1304–5.ArticlePubMedPMC
- 6. Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol 2003;2:28. ArticlePubMedPMC
- 7. Ben Sahra I, Le Marchand-Brustel Y, Tanti JF, Bost F. Metformin in cancer therapy: a new perspective for an old antidiabetic drug? Mol Cancer Ther 2010;9:1092–9.ArticlePubMedPDF
- 8. Spratt DE, Zhang C, Zumsteg ZS, Pei X, Zhang Z, Zelefsky MJ. Metformin and prostate cancer: reduced development of castration-resistant disease and prostate cancer mortality. Eur Urol 2013;63:709–16.ArticlePubMedPMC
- 9. Wright JL, Stanford JL. Metformin use and prostate cancer in Caucasian men: results from a population-based case-control study. Cancer Causes Control 2009;20:1617–22.ArticlePubMedPMC
- 10. Margel D, Urbach DR, Lipscombe LL, Bell CM, Kulkarni G, Austin PC, et al. Metformin use and all-cause and prostate cancer-specific mortality among men with diabetes. J Clin Oncol 2013;31:3069–75.ArticlePubMed
- 11. Kaushik D, Karnes RJ, Eisenberg MS, Rangel LJ, Carlson RE, Bergstralh EJ. Effect of metformin on prostate cancer outcomes after radical prostatectomy. Urol Oncol 2014;32:43.e1–7. ArticlePubMedPMC
- 12. Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009;52:1766–77.ArticlePubMedPDF
- 13. Cantagallo A, Delli Castelli M. Cost-free prevention to asymptomatic bacteriuria in diabetic women: two hands, two towels. Diabetes Care 2001;24:412–4.Article
- 14. Szablewski L. Diabetes mellitus: influences on cancer risk. Diabetes Metab Res Rev 2014;30:543–53.ArticlePubMed
- 15. Murtola TJ, Vihervuori VJ, Lahtela J, Talala K, Taari K, Tammela TL, et al. Fasting blood glucose, glycaemic control and prostate cancer risk in the Finnish Randomized Study of Screening for Prostate Cancer. Br J Cancer 2018;118:1248–54.ArticlePubMedPMCPDF
- 16. Grossmann M, Wittert G. Androgens, diabetes and prostate cancer. Endocr Relat Cancer 2012;19:F47–62.ArticlePubMed
- 17. Tande AJ, Platz EA, Folsom AR. The metabolic syndrome is associated with reduced risk of prostate cancer. Am J Epidemiol 2006;164:1094–102.ArticlePubMed
- 18. Murtola TJ, Tammela TL, Lahtela J, Auvinen A. Antidiabetic medication and prostate cancer risk: a population-based case-control study. Am J Epidemiol 2008;168:925–31.ArticlePubMed
- 19. Rodriguez C, Patel AV, Mondul AM, Jacobs EJ, Thun MJ, Calle EE. Diabetes and risk of prostate cancer in a prospective cohort of US men. Am J Epidemiol 2005;161:147–52.ArticlePubMed
- 20. Chen X, Li C, He T, Mao J, Li C, Lyu J, et al. Metformin inhibits prostate cancer cell proliferation, migration, and tumor growth through upregulation of PEDF expression. Cancer Biol Ther 2016;17:507–14.ArticlePubMedPMC
- 21. Wang CP, Lehman DM, Lam YF, Kuhn JG, Mahalingam D, Weitman S, et al. Metformin for reducing racial/ethnic difference in prostate cancer incidence for men with type II diabetes. Cancer Prev Res (Phila) 2016;9:779–87.ArticlePubMedPMCPDF
- 22. Wang CP, Hernandez J, Lorenzo C, Pollock B, Lehman DM. Metformin effects on high- vs. low-grade prostate cancer. Diabetes 2013;62(Suppl 1):LB31.
- 23. Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 2009;32:1620–5.ArticlePubMedPMC
- 24. Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006;29:254–8.ArticlePubMedPDF
- 25. Bowker SL, Yasui Y, Veugelers P, Johnson JA. Glucose-lowering agents and cancer mortality rates in type 2 diabetes: assessing effects of time-varying exposure. Diabetologia 2010;53:1631–7.ArticlePubMedPDF
- 26. Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, Bilo HJ. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care 2010;33:322–6.ArticlePubMedPMC
- 27. Ben Sahra I, Laurent K, Giuliano S, Larbret F, Ponzio G, Gounon P, et al. Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res 2010;70:2465–75.ArticlePubMedPDF
- 28. Oliveras-Ferraros C, Vazquez-Martin A, Menendez JA. Genome-wide inhibitory impact of the AMPK activator metformin on [kinesins, tubulins, histones, auroras and polo-like kinases] M-phase cell cycle genes in human breast cancer cells. Cell Cycle 2009;8:1633–6.ArticlePubMed
Citations
Citations to this article as recorded by

 , Minsun Song2,*
, Minsun Song2,* , Jiwon Shinn1
, Jiwon Shinn1 , Hun-Sung Kim1,3
, Hun-Sung Kim1,3


