ABSTRACT
- The global response to the COVID-19 pandemic has led to rapid vaccine development and distribution. As vaccination efforts continue, concerns have arisen regarding potential adverse events associated with COVID-19 vaccination. This article examines emerging evidence on adverse events, including myocarditis, pericarditis, and thrombotic complications, in relation to COVID-19 vaccination. Reports of myocarditis and pericarditis cases following messenger RNA vaccines have sparked interest, with discussions revolving around potential mechanisms and genetic predispositions. The contrasting findings on pericarditis risk postvaccination highlight the complexity of studying this phenomenon. Thrombotic events, particularly vaccine-induced thrombotic thrombocytopenia, have garnered attention, prompting investigations into antibody responses and mechanisms. This article underscores the importance of ongoing research, collaboration, and data analysis for accurately understanding adverse events. While the COVID-19 vaccination campaign may have ended, it is still vital to maintain vigilance, collect comprehensive data and foster interdisciplinary collaboration to uphold vaccine safety and steer public health strategies in the upcoming period.
-
Keywords: COVID-19; Vaccination; Cardiovascular diseases; Adverse cardiac events
INTRODUCTION
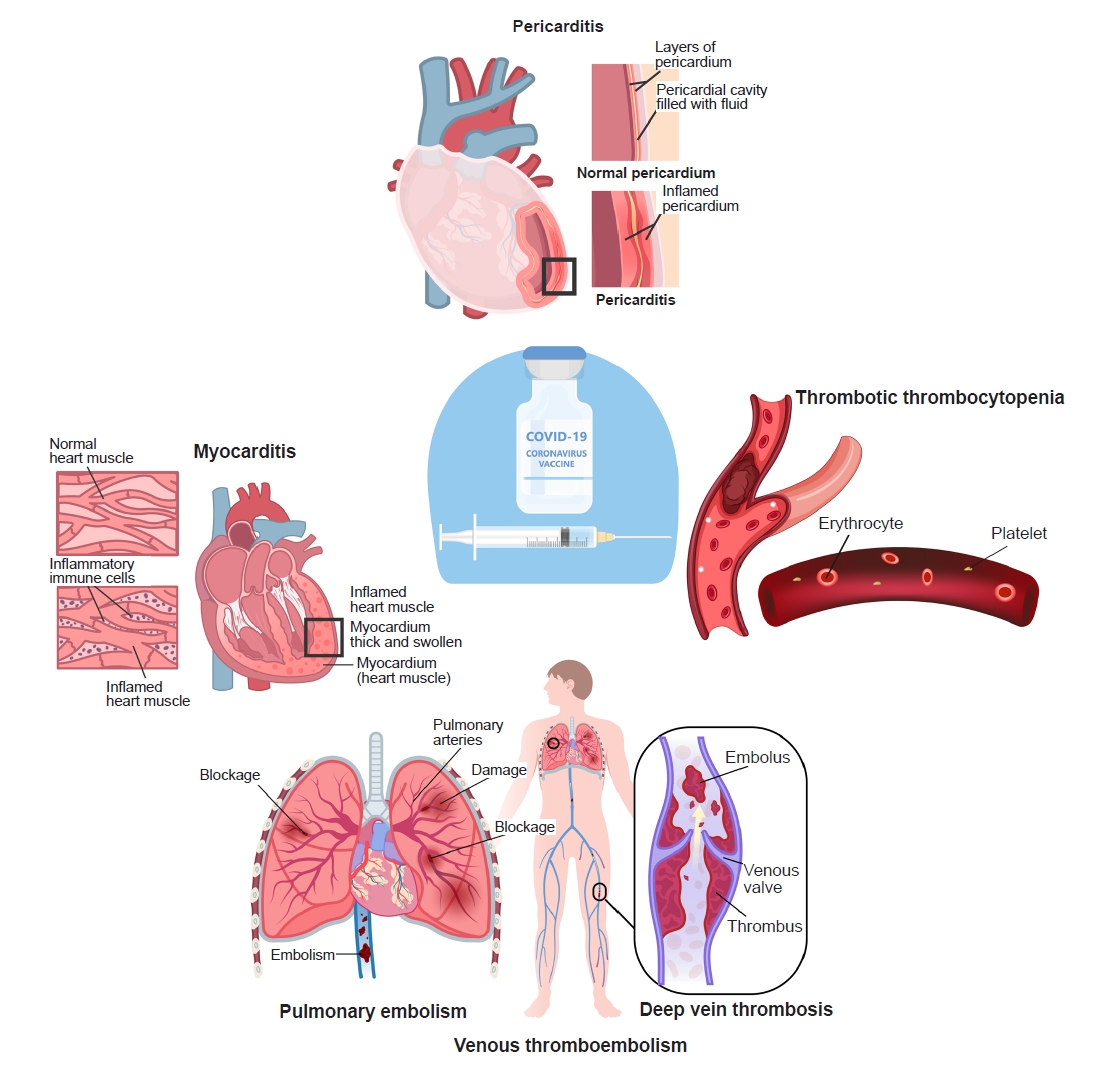
- The initiation of the COVID-19 vaccination in December 2020 in the United States and various other Western countries, with subsequent commencement in Korea in February 2021, engendered notable domestic and global concerns. While typical vaccination-related manifestations were documented, there were also reports of serious adverse events emerging. The initial vaccines utilized adenoviral vectors, raising concerns about thrombosis events, while the widespread distribution of messenger RNA (mRNA) vaccines has brought issues such as myocarditis and pericarditis to the forefront. It is also noteworthy that instances of vaccine-related myocarditis displayed varying clinical outcomes between countries and ethnicities [1,2], and in particular, that more adverse events occurred within the Korean context [3]. The ongoing COVID-19 pandemic has led to an unprecedented global effort to develop and distribute vaccines to combat the spread of the virus. As vaccination campaigns progress, it is crucial to closely monitor and assess potential adverse events associated with the vaccines. Among the reported adverse events, myocarditis, pericarditis, and thrombotic complications have garnered attention due to their potential links to COVID-19 vaccination (Fig. 1). This review delves into the emerging evidence surrounding these adverse events and explores their possible associations with different types of vaccines.
MYOCARDITIS
- There is a substantial body of evidence indicating a potential association between COVID-19 vaccination and the development of myocarditis. Myocarditis and pericarditis are known complications of mRNA vaccines, especially in young adults and teen boys aged 12 to 17 years [4], with the highest observed incidence within 2 to 7 days after the second dose at a rate of 3.5 to 140 per million doses [5,6]. The incidence of myocarditis is rare, and myocarditis occurs much more frequently following COVID-19 infection than following vaccination [4]. Even in the group at highest risk, boys aged 12 to 17 years, the risk of myocarditis was 1.8 to 5.6 times higher after SARS-CoV-2 infection than after vaccination [4]. Myocarditis was estimated to develop 1 to 10 per million persons in the month following vaccination, which was substantially lower than observed after SARS-CoV-2 infection [2]. Cardiovascular events following vaccination are rare and should be considered alongside the overall benefits of COVID-19 vaccination [4,7]. General myocarditis, especially viral myocarditis, is known to have an annual incidence rate of 1 to 10 cases per 100,000 individuals. Following mRNA COVID-19 vaccination, an incidence rate of 1.4 to 5.0 cases per 100,000 individuals within 7 to 42 days has been reported [8]. A population-based cohort study in Denmark [9], analyzing myocarditis and pericarditis occurring within 28 days of vaccination in 4,931,775 vaccine recipients from October 2020 to October 2021, revealed an adjusted risk ratio of 1.34 (95% confidence interval [CI], 0.90–2.00) for myocarditis following BNT162b2 vaccination and 3.92 (95% CI, 2.30–6.68) following mRNA-1273 vaccination. In a self-controlled case series within the cohort study, the rate ratio was 1.48 (95% CI, 0.93–2.36) for BNT162b2 vaccine recipients and 6.25 (95% CI, 2.83–13.82) for mRNA-1273 vaccine recipients. An analysis of data from the largest healthcare organization in Israel (Clalit Health Services) [1], demonstrated a myocarditis incidence rate of 2.13 cases per 100,000 individuals within 42 days after the first dose of mRNA vaccines. Notably, the highest myocarditis incidence rate was observed in men aged 16 to 29 years, reaching 10.69 cases per 100,000 individuals, with rates of 4.12 and 0.23 cases per 100,000 in men and women overall, respectively. An analysis of data from the Ministry of Health of Israel [10] revealed that myocarditis occurrence after BNT162b2 vaccination exceeded the expected frequency based on 2017–2019 incidence rates. The analysis, including the 21 days after the first dose and 30 days after the second dose, showed a post–first-dose rate ratio of 1.42 (95% CI, 0.92–2.10) and a post–second-dose rate ratio of 5.34 (95% CI, 4.48–6.40) compared to the expected frequency in unvaccinated individuals. Data analysis of reports submitted to the national passive reporting system, Vaccine Adverse Event Reporting System (VAERS), in the United States [5] revealed 1,626 cases of myocarditis following mRNA vaccine administration from December 2021 to August 2022. The incidence of myocarditis within 7 days of vaccination exceeded the expected frequency based on 2017–2019 claims data in multiple age and sex strata. Notably, the frequency of myocarditis following vaccination was highest among 12- to 15-year-old boys, with 70.7 cases per million BNT162b2 doses administered, and ranged from 52.4 to 105.9 cases per million doses among other age groups and vaccines. These four studies consistently reported relatively consistent results—namely, increased rates following the second dose, higher rates in young men, and rates exceeding the expected frequency in unvaccinated individuals. A notable difference in Denmark's report [9] was the relatively lower adjusted hazard ratio (aHR) following BNT162b2 vaccination, which may be attributed to the specificity of risk in patients with no other risk factors apart from vaccination. Although different studies used varying risk intervals between 7 and 42 days after vaccination, all reported higher rates of myocarditis during the risk interval than expected. Although consistent results have been reported regarding an increased rate of myocarditis following the second dose compared to the first, interpreting this as a dose-response relationship is challenging. With ongoing third-dose vaccinations, further research is needed to determine whether the incidence of myocarditis remains higher after the second dose. However, this possibility is unlikely, and immunological attenuation may provide a more plausible explanation. SARS-CoV-2 mRNA vaccines contain nucleoside-modified mRNA encoding the viral spike glycoprotein, which, when administered, generates adaptive immune responses to produce immunoglobulin G (IgG) antibodies against the viral spike protein, facilitating virus neutralization. While some RNA can stimulate the innate immune system on its own, mRNA vaccines underwent nucleoside modification to reduce innate immunogenicity. Nonetheless, in individuals with certain genetic predispositions, an immune response to mRNA could trigger proinflammatory cascades, leading to systemic reactions, including myocarditis [11].
- In a large Israeli cohort study [1], only one out of 54 patients with COVID-19 vaccine–related myocarditis (VRM) developed cardiogenic shock requiring extracorporeal membrane oxygenation support. A US study involving 40 hospitals [12] reported no readmissions or deaths among COVID-19 VRM patients, with all patients discharged within a median of 2 days (interquartile range, 2–3 days). In a Korean nationwide study from our team [3], we observed 95 severe COVID-19 VRM cases (19.8% of total VRM), including 36 cases of fulminant myocarditis and 21 deaths. Additionally, we identified eight cases of sudden cardiac death confirmed through autopsy. Given our extensive dataset of over 44 million individuals, it is possible that our study observed more deaths than studies with smaller populations. However, the largest cohort in the US [5], encompassing 192,405,448 individuals, reported no COVID-19 VRM-related deaths. The discrepancy between our findings and those in the United States may be attributed to differences in case reporting systems. Most US studies utilized the VAERS, a passive reporting system susceptible to both underreporting and overreporting. In contrast, the Korean government established a comprehensive reporting system for adverse events before COVID-19 vaccination, along with a national compensation system for related medical expenses. Given that VRM is a legally mandated reportable adverse reaction to COVID-19 vaccination in Korea, the risk of underreporting is minimized. The Korean government also established a causality assessment committee to review and confirm vaccination-associated cases, further reducing the potential for overreporting of VRM.
- The exact mechanisms underlying mRNA vaccine-induced myocarditis are still not fully understood. Nevertheless, some reports indicate that the mRNA-1273 vaccine may initiate a strong CD4 cytokine response, particularly involving type 1 helper T (Th1) cells. CD4 cells have been suggested as a possible contributor to the onset of myocarditis [13,14]. The detailed mechanisms warrant further clinical and basic research. Myocarditis and perimyocarditis may be caused by the direct invasion of cardiomyocytes by SARS-CoV-2 [15] and an inflammatory response. However, the fact that myocarditis occurs after vaccination, in the absence of live viruses, also points to an immune-mediated phenomenon and molecular mimicry between the spike protein (present in infection and after vaccination) and autoantigens in genetically predisposed persons, as confirmed by the discovery of autoantibodies in some patients [11]. New evidence suggests the role of endogenous autoantibodies against interleukin-1 receptor antagonist (IL-1RA) and hyperphosphorylated IL-1RA in triggering myocarditis in young male adults [16]. Further investigation into the mechanism of vaccine-related myocarditis and the development of effective treatment strategies are warranted.
PERICARDITIS
- Rather than myocarditis, a notable proportion of patients have experienced chest pain after receiving the COVID-19 vaccine, leading to a diagnosis of pericarditis. Some of these instances have posed more substantial treatment challenges and involved extended recovery periods in contrast to typical viral pericarditis cases.
- A study analyzing cases of pericarditis following COVID-19 vaccine administration through medical records from 40 hospitals in the United States [12] reported a total of 37 cases (15 cases after the first dose and 22 cases after the second dose) out of a total of 2,000,287 doses administered. This indicated a significantly higher incidence of pericarditis compared to the prevaccination period (prevaccination, 49.1%; postvaccination, 78.8%). A self-controlled case series conducted in the United Kingdom [2] reported a total of 1,574 cases of pericarditis between December 2020 and August 2021. Among these, 356 cases occurred within 28 days after mRNA vaccine administration, with 188 cases occurring in COVID-19–infected patients and 154 cases occurring before vaccine administration. Hospitalizations and deaths due to pericarditis increased within 14 days after COVID-19 infection (incidence rate ratio [IRR], 3.81; 95% CI, 1.90–7.63), but there was no increase in hospitalizations or deaths due to pericarditis after vaccine administration. Instead, there was a lower risk (ChAdOx1 first dose: IRR, 0.59 [95% CI, 0.37–0.94]; BNT162b2 first dose: IRR, 0.46 [95% CI, 0.24–0.90]). The risk of pericarditis within 28 days after COVID-19 infection was elevated (IRR, 2.79; 95% CI, 1.80–4.32), but the risk was lower within 28 days after ChAdOx1 first dose (IRR, 0.74; 95% CI, 0.59–0.92). A case-control study comparing hospital control participants and patients hospitalized with carditis after BNT162b2 and Sinovac-CoronaVac vaccine administration in Hong Kong [17] showed an adjusted odds ratio of 7.78 (95% CI, 3.76–16.13) for carditis within 14 days after BNT162b2 vaccine administration. In the subgroup analysis, the adjusted odds ratio was 9.29 (95% CI, 3.94–21.91) for myocarditis and 1.06 (95% CI, 0.35–3.22) for pericarditis. The results from the studies above show somewhat contradictory findings. An analysis of medical records from 40 US hospitals following vaccine administration [12] indicated an increased incidence of pericarditis after vaccine initiation compared to the prevaccination period. However, other studies did not find an increased rate of pericarditis following vaccine administration. This could be interpreted as reflecting a specific association in patients without other risk factors, but caution is needed due to the low incidence of pericarditis itself and the possibility of its occurrence in the absence of vaccine-related factors. In the US study, there were 193 cases of pericarditis after the first dose and 374 cases after the second dose, but interpreting this in terms of a dose-response relationship is difficult. Considering the potential mechanisms of myocarditis and pericarditis, this seems to be driven more by immunological modulation rather than a straightforward dose-response relationship. Other research results outside the US [2,17] showed that the rate of pericarditis occurrence did not increase after vaccine administration.
- SARS-CoV-2 mRNA vaccines contain nucleoside-modified mRNA encoding the viral spike glycoprotein of SARS-CoV-2. Delivered within lipid nanoparticles, these vaccines prompt the human cells to produce the spike protein, initiating adaptive immune responses that generate IgG antibodies targeting the viral spike protein for virus neutralization. While some RNA can independently stimulate the innate immune system, nucleoside modifications were introduced to mRNA vaccines to minimize innate immunogenicity. Nevertheless, in individuals with specific genetic predispositions, an immune response to mRNA can trigger proinflammatory pathways, potentially leading to systemic reactions like myocarditis and pericarditis. It is acknowledged that side effects resembling myocarditis and pericarditis have been observed following smallpox vaccination, although the underlying mechanisms differ from those of mRNA vaccines. This underscores the established understanding that vaccines designed to elicit immune responses can carry a risk of myocarditis and pericarditis [18].
VACCINE-INDUCED THROMBOTIC THROMBOCYTOPENIA
- Vaccine-induced thrombotic thrombocytopenia (VITT) has emerged as a rare side effect of adenoviral vector–based vaccines against COVID-19 and has been most frequently reported after the use of the ChAdOx1 vaccine [19]. One of the first reports of COVID-19 VITT [20], which was released on April 9, 2021, involved five healthcare workers aged 32 to 54 years 7 to 10 days after receiving their first dose. The incidence was five out of 130,000 vaccinated persons in that report. In the same issue of the New England Journal of Medicine, a German and Austrian group [21] also reported 11 patients (nine women aged 22–49 years) with VITT after ChAdOx1 nCov-19 vaccination. They reported nine cases of cerebral venous thrombosis, three cases of splanchnic vein thrombosis, three cases of pulmonary embolism (PE), and four cases of other thromboses. Six of them died and five developed disseminated intravascular coagulation. Soon afterwards, a UK group [22] reported 23 VITT patients, 22 of whom tested positive for platelet factor 4 (PF4), including one equivocal test. A nonheparin anticoagulant agent and intravenous immunoglobulin were recommended for those patients. Nonetheless, intravenous immunoglobulin therapy might obscure the capacity of anti-PF4/heparin antibodies to interact with and trigger platelet activation in the presence of heparin, potentially yielding erroneous negative outcomes in immunoassay functional tests. A case of VITT and PE was reported 13 days after receiving the single dose of Ad26 vaccines [19]. Additionally, reports have surfaced regarding instances of cerebral venous sinus thrombosis (CVST) in the United States during the period from March 2 to April 21, 2021. By April 12, 2021, roughly 7 million doses of the Ad26.COV2.S vaccine had been administered in the United States, and six cases of CVST accompanied by thrombocytopenia were identified among the vaccine recipients. Consequently, a temporary nationwide halt to vaccination with this product was instituted on April 13, 2021 [23].
- VITT has been reported after mRNA vaccines as well as adenoviral vector vaccines. Al-Rasbi et al. [24] presented a 37-year-old man with myocarditis, pulmonary edema, and pulmonary hemorrhage 12 days after receiving the first dose of BNT162b2 mRNA COVID-19 vaccination. He responded favorably to a 5-day course of intravenous methylprednisolone and immunoglobulin. A case series study [25] also reported a short-term risk of PE among French residents aged 75 years or older after receipt of the BNT162b2 mRNA injection. A study in Italy [26] also reported a combination of acute exacerbation of interstitial lung disease and PE in an elderly patient after booster mRNA vaccination against COVID-19. A study in Saudi Arabia [27] also reported a 78-year-old man who developed PE 1 day after receiving the second dose of the BNT162b2 vaccine. Another case of a healthy 24-year-old young man with PE due to the BNT162b2 vaccine [28] also reported that his symptoms started 6 hours after administration of the second dose of the vaccine.
- Antibodies directed against PF4, also known as CXCL4, are implicated in the pathogenesis of VITT. These antibodies, which belong to the IgG class, interact with platelet FcγIIa receptors with relatively low affinities, leading to platelet activation [21,29]. Ongoing research aims to elucidate the mechanisms by which the vaccines in question induce the production of new antibodies or activate preexisting ones. A developing model proposes that the vaccine initiates the generation of neoantigens as an initial step, followed by a systemic inflammatory response as a secondary step. This dual process seems to contribute to the formation of anti-PF4 antibodies. Noteworthy components of the vaccine that have the capacity to bind to PF4 and induce structural changes, thus creating neoantigens, include viral proteins originating from the HEK3 cell line and free DNA. Preliminary investigations suggest that binding of adenoviral hexon proteins to PF4 may play a role in this process [29–31].
- The incidence of VITT in Korea is very low according to a report from Korea Disease Control and Prevention Agency (KDCA) [32], and VITT was considered in 214 cases in Korea, yet it has been definitively diagnosed in only four cases to date: two men in their 30s with cerebral vein thrombosis, one woman in her 70s with deep vein thrombosis, and one man in his 60s with pulmonary arterial thromboembolism.
VENOUS THROMBOEMBOLISM
- In a UK study [33] from December 2020 to April 2021, involving 19,608,008 ChAdOx1 vaccine recipients, 9,513,625 BNT162b2 vaccine recipients, and 1,758,095 COVID-19 cases, venous thromboembolism risk increased 8 to 14 days post-ChAdOx1 vaccination (IRR, 1.10; 95% CI, 1.02–1.18). COVID-19 patients also had a substantial risk increase (IRR, 13.86; 95% CI, 12.76–15.05), whereas BNT162b2 vaccination did not significantly elevate the risk. Venous thromboembolism after ChAdOx1 vaccination often coincided with thrombocytopenia (IRR, 1.34; 95% CI, 0.99–1.83). Analyzing data from the EudraVigilance database [34], BNT162b2 vaccines had 33 adverse reactions per million doses, while ChAdOx1 vaccines had 151 per million. BNT162b2 recipients had lower thrombosis rates than unvaccinated individuals, but ChAdOx1 recipients had a higher PE risk ratio (18–64 years, 18.8 [95% CI, 4.3–5.1]; >65 years, 4.0 [95% CI, 3.7–4.2]). Analyzing data until June 23, 2021, for ChAdOx1, BNT162b2, and Ad26 vaccines, Cari et al. [35] found that thrombotic events of the Ad26 vaccine were comparable to ChAdOx1's, both surpassing BNT162b2, across age groups (18–64 and >65 years).
- A study based on data from eight US health plans [36], spanning from December 14, 2020, to January 26, 2021, analyzed adverse reactions following the first and second doses of BNT162b2 and mRNA-1273 vaccines within 21 days. While the adjusted rate ratio for venous thromboembolism was 1.16 (95% CI, 1.00–1.34), the two-sided P-value was not statistically significant at 0.05. In a study analyzing thrombotic reactions among BNT162b2 vaccine recipients aged 75 and above in France [25], after first and second doses, PE occurrence showed no increase post–first dose (relative incidence [RI], 0.85; 95% CI, 0.75–0.96) and a slight increase post–second dose (RI, 1.10; 95% CI, 0.95–1.26). Based on a national prospective cohort study in Scotland [37], a nested incident-matched case-control study found no association between deep vein thrombosis occurrence (adjusted rate ratio [aRR], 1.21; 95% CI, 0.95–1.54) or PE occurrence (aRR, 0.78; 95% CI, 0.63–0.96) and ChAdOx1 vaccine administration. Similarly, there was no association between deep vein thrombosis occurrence (aRR, 0.79; 95% CI, 0.56–1.11) or PE occurrence (aRR, 0.35; 95% CI, 0.26–0.48) and BNT162b2 vaccine administration. In a retrospective analysis of Mayo Clinic medical records in the United States [38], venous thromboembolism occurrence within 90 days following the first vaccine dose was compared to the 90 days prior to vaccination. The BNT162b2 (aHR, 1.00; 95% CI, 0.87–1.15), mRNA-1273 (aHR, 1.02; 95% CI, 0.87–1.19), and Ad.26 (aHR, 0.97; 95% CI, 0.63–1.50) vaccines did not show an increased risk of venous thromboembolism. In a population-based cohort study in Spain [39], deep vein thrombosis occurrence (standardized incidence rate [SIR], 0.89; 95% CI, 0.65–1.22) and PE occurrence (SIR, 0.78; 95% CI, 0.52–1.16) did not increase following ChAdOx1 vaccination. Similarly, deep vein thrombosis (SIR, 1.03; 95% CI, 0.89–1.19) and PE (SIR, 0.84; 95% CI, 0.84–1.20) did not increase after the first dose of BNT162b2, but a slight increase in PE was observed after the first dose (SIR, 1.25; 95% CI, 1.07–1.46). Notably, the incidence of both deep vein thrombosis (SIR, 4.68; 95% CI, 4.07–5.38) and PE (SIR, 17.86; 95% CI, 16.37–19.50) increased significantly after COVID-19 infection. A systematic review of eight studies [40], including two randomized controlled trials, five large-scale case-control series, and one large prospective cohort study, indicated that mRNA vaccines did not increase the risk of venous thromboembolism (RR, 0.48–1.20). In a population-based cohort study conducted in Denmark and Norway [41], individuals aged 18 to 65 years who received the ChAdOx1 vaccine were compared to the general populations of Denmark (2016–2018) and Norway (2018–2019). The study findings revealed a significant increase in venous thromboembolism cases among vaccinated people. Based on the current evidence, it is suggested that there is a slight increase in the risk of venous thromboembolism with adenovirus vector vaccines, while such a risk is less evident with mRNA vaccines.
AREA OF UNCERTAINTY
- Acute myocardial infarction
- Studies investigating myocardial infarction risk have yielded mixed results, emphasizing the need for thorough assessments across different vaccines and populations. In an Israeli study [42], observations were made up to 42 days after administering the BNT162b2 mRNA vaccine. Each group consisted of 890,000 individuals, with 59 myocardial infection events in the vaccine group and 60 in the control group; this yielded a risk ratio of 1.07 (95% CI, 0.74–1.60), with no significant difference. Research from medical institutions including the Kaiser Permanente group in the United States [36] examined the effects of BNT162b2 and mRNA-1273 vaccines within 21 days of administration. Among 118.4 million doses administered, the number of myocardial infarction occurrences was 935 versus 1,030 (RR, 1.02; 95% CI, 0.89–1.18), indicating no increase in postvaccination events. A study in France [25] observed patients aged 75 and above after initial BNT162b2 mRNA vaccine administration for 14 days. Among 3.2 million patients who received at least two doses, 538 incidents were observed, with an RR of 1.08 (95% CI, 0.97–1.21), indicating no significant increase in myocardial infarction occurrence. A study published in BMJ [41] examined arterial events, venous thromboembolism, thrombocytopenia, and bleeding occurring within 28 days after the first dose of the ChAdOx1 vaccine in Denmark and Norway, spanning from February to March 2021. Using a population-based cohort approach, the study compared event rates among vaccine recipients to the general population's expected event rates. While venous thromboembolism showed an association with vaccine administration, arterial events, including acute myocardial infection, did not increase. For acute myocardial infarction, the standardized morbidity ratio was 1.09 (95% CI, 0.66–1.68), with an expected number of 18 and an observed number of 20, showing no significant difference between groups.
- The occurrence of myocardial infarction does not seem to relate to vaccination. In fact, the occurrence rate of myocardial infarction tends to increase in patients with underlying conditions like old age, hypertension, and diabetes. While myocardial infarction can occur after vaccination, acute myocardial infarction has been occurring at a consistent rate even before the era of COVID-19 vaccines. In recent years, its incidence rate has slightly increased. According to the Health Insurance Review and Assessment Service (HIRA) data [43], there has been an increase in myocardial infarction cases in Korea from 93,475 in 2016 to 121,169 in 2020, though variations exist between countries, with a decreasing trend in the United States. This increase is attributed to the rising prevalence of risk factors such as hypertension, diabetes, and dyslipidemia, which are associated with coronary artery disease.
- The occurrence of ischemic heart disease as a hypersensitivity reaction following vaccination or medication is referred to as Kounis syndrome. It involves the release of mediators such as histamine, thromboxane, prostaglandins, leukotrienes, and platelet-activating factors from mast cells in response to allergic reactions, triggering vasospasms and coronary artery constriction. The syndrome is classified into three types. While cases of Kounis syndrome postvaccination have been reported, they are rare and fall within the realm of hypersensitivity reactions rather than direct vaccine side effects [44].
- Heart failure
- There is no documented research linking heart failure to COVID-19 vaccination. However, the emergence of myocarditis after mRNA COVID-19 vaccination is recognized, as we already mentioned in the present review. While typically mild, with no manifestation of heart failure symptoms, there have been some severe cases accompanied by acute heart failure [45]. Following myocarditis, it is conceivable for dilated cardiomyopathy, a form of heart failure, to develop as a sequela. This progression is often termed as "inflammatory cardiomyopathy." However, no current evidence suggests that myocarditis resulting from vaccination follows such a trajectory. The present understanding is that patients recover without significant lingering effects. While there has been no documented association between COVID-19 vaccination and heart failure, there have been recurrent reports of myocarditis following the administration of the COVID-19 vaccine. Domestic causality assessments have acknowledged this association. Although the long-term consequences of vaccine-associated myocarditis are not yet known, it is established that myocarditis caused by viral infections can progress to dilated cardiomyopathy and result in the formation of scar tissue within the heart, potentially leading to heart failure. Therefore, there exists a possibility that vaccine-associated myocarditis might demonstrate similar long-term sequelae upon prolonged observation.
- The SARS-CoV-2 mRNA vaccine contains nucleoside-modified mRNA coding for the SARS-CoV-2 viral spike glycoprotein. This is encapsulated in lipid nanoparticles, and once administered, the mRNA is introduced into human cells. This prompts the cells to produce the spike protein, which in turn stimulates the adaptive immune response, facilitating the production of IgG antibodies against the viral spike protein, thereby conferring neutralizing ability against the virus. Some RNA inherently stimulates the innate immune system, leading to the premature degradation of mRNA before it reaches the target cells. As a result, the mRNA vaccines underwent nucleoside modification to reduce this innate immunogenicity. Nevertheless, in certain individuals with genetic predispositions, an immune response may be triggered against the mRNA itself, initiating proinflammatory cascades that could potentially explain some of the vaccine-associated inflammatory complications, including myocarditis and pericarditis [11]. Viral myocarditis typically undergoes three stages of reaction before reaching the recovery phase. However, in some cases, if infected cells are not entirely eliminated or immune cells with auto-reactive capabilities persist within the myocardium, chronic inflammation can occur and progress into dilated cardiomyopathy [46]. If postvaccination, the immune system activated by the mRNA itself does not stabilize after a temporary inflammatory response and continues to induce myocardial damage, there is a potential for progression similar to that observed after viral myocarditis, eventually leading to heart failure. The occurrence of heart failure potentially associated with vaccination can be largely attributed to acute heart failure due to myocarditis. Additionally, if it occurs upon subsequent tracking, it could be considered a transition from recovered myocarditis to chronic heart failure. Beyond these two scenarios, other causes are difficult to postulate.
CONCLUSIONS
- The occurrence of adverse events subsequent to COVID-19 vaccination, such as myocarditis, pericarditis, and thrombotic incidents, has spurred extensive research endeavors aimed at investigating potential associations and underlying mechanisms. The existing body of evidence presents a multifaceted scenario, with certain studies indicating heightened risks and others failing to establish significant correlations. Sustained vigilance, robust data collection, and thorough investigations remain imperative to gain a comprehensive understanding of the connections between COVID-19 vaccination and adverse events.
- In forthcoming vaccination campaigns, it is crucial to take into account various factors, encompassing vaccine types, patient demographics, and preexisting risk factors, when assessing adverse events. Collaborative efforts among healthcare professionals, researchers, and regulatory authorities are pivotal in making well-informed decisions and ensuring the safety of vaccine recipients. Ongoing research endeavors will further illuminate the intricacies of adverse events associated with COVID-19 vaccination and provide valuable insights to shape future vaccination strategies.
ARTICLE INFORMATION
-
Ethics statements
Not applicable.
-
Conflicts of interest
The authors have no conflicts of interest to declare.
-
Funding
None.
-
Author contributions
Conceptualization: all authors; Investigation: JYC; Methodology: KHK; Project administration: KHK; Resources: KHK; Supervision: KHK; Validation: KHK; Visualization: JYC; Writing–original draft: JYC; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Fig. 1.COVID-19 vaccination–related cardiovascular complications.
REFERENCES
- 1. Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, et al. Myocarditis after COVID-19 vaccination in a large health care organization. N Engl J Med 2021;385:2132–9.ArticlePubMedPMC
- 2. Patone M, Mei XW, Handunnetthi L, Dixon S, Zaccardi F, Shankar-Hari M, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med 2022;28:410–22.ArticlePubMedPMCPDF
- 3. Cho JY, Kim KH, Lee N, Cho SH, Kim SY, Kim EK, et al. COVID-19 vaccination-related myocarditis: a Korean nationwide study. Eur Heart J 2023;44:2234–43.ArticlePubMedPMCPDF
- 4. Block JP, Boehmer TK, Forrest CB, Carton TW, Lee GM, Ajani UA, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination: PCORnet, United States, January 2021-January 2022. MMWR Morb Mortal Wkly Rep 2022;71:517–23.ArticlePubMedPMC
- 5. Oster ME, Shay DK, Su JR, Gee J, Creech CB, Broder KR, et al. Myocarditis cases reported after mRNA-Based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA 2022;327:331–40.ArticlePubMedPMC
- 6. Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect 2022;28:202–21.ArticlePubMedPMC
- 7. Pillay J, Gaudet L, Wingert A, Bialy L, Mackie AS, Paterson DI, et al. Incidence, risk factors, natural history, and hypothesised mechanisms of myocarditis and pericarditis following COVID-19 vaccination: living evidence syntheses and review. BMJ 2022;378:e069445.ArticlePubMedPMC
- 8. Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol 2022;19:75–7.ArticlePubMedPMCPDF
- 9. Husby A, Hansen JV, Fosbol E, Thiesson EM, Madsen M, Thomsen RW, et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ 2021;375:e068665.ArticlePubMedPMC
- 10. Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, Nadir E, et al. Myocarditis after BNT162b2 mRNA vaccine against COVID-19 in Israel. N Engl J Med 2021;385:2140–9.ArticlePubMedPMC
- 11. Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation 2021;144:471–84.ArticlePubMedPMC
- 12. Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson IV, Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA 2021;326:1210–2.ArticlePubMedPMC
- 13. Vdovenko D, Eriksson U. Regulatory role of CD4+ T cells in myocarditis. J Immunol Res 2018;2018:4396351. ArticlePubMedPMCPDF
- 14. Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 2020;383:2427–38.ArticlePubMedPMC
- 15. Hanson PJ, Liu-Fei F, Ng C, Minato TA, Lai C, Hossain AR, et al. Characterization of COVID-19-associated cardiac injury: evidence for a multifactorial disease in an autopsy cohort. Lab Invest 2022;102:814–25.ArticlePubMedPMCPDF
- 16. Thurner L, Kessel C, Fadle N, Regitz E, Seidel F, Kindermann I, et al. IL-1RA antibodies in myocarditis after SARS-CoV-2 vaccination. N Engl J Med 2022;387:1524–7.ArticlePubMedPMC
- 17. Lai FT, Li X, Peng K, Huang L, Ip P, Tong X, et al. Carditis after COVID-19 vaccination with a messenger RNA vaccine and an inactivated virus vaccine: a case-control study. Ann Intern Med 2022;175:362–70.ArticlePubMedPMC
- 18. Cassimatis DC, Atwood JE, Engler RM, Linz PE, Grabenstein JD, Vernalis MN. Smallpox vaccination and myopericarditis: a clinical review. J Am Coll Cardiol 2004;43:1503–10.ArticlePubMed
- 19. Curcio R, Gandolfo V, Alcidi R, Giacomino L, Campanella T, Casarola G, et al. Vaccine-induced massive pulmonary embolism and thrombocytopenia following a single dose of Janssen Ad26.COV2.S vaccination. Int J Infect Dis 2022;116:154–6.ArticlePubMedPMC
- 20. Schultz NH, Sorvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2124–30.ArticlePubMed
- 21. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021;384:2092–101.ArticlePubMedPMC
- 22. Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2202–11.ArticlePubMedPMC
- 23. See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA 2021;325:2448–56.ArticlePubMedPMC
- 24. Al-Rasbi S, Al-Maqbali JS, Al-Farsi R, Al Shukaili MA, Al-Riyami MH, Al Falahi Z, et al. Myocarditis, pulmonary hemorrhage, and extensive myositis with rhabdomyolysis 12 days after first dose of Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine: a case report. Am J Case Rep 2022;23:e934399.ArticlePubMedPMC
- 25. Jabagi MJ, Botton J, Bertrand M, Weill A, Farrington P, Zureik M, et al. Myocardial infarction, stroke, and pulmonary embolism after BNT162b2 mRNA COVID-19 vaccine in people aged 75 years or older. JAMA 2022;327:80–2.ArticlePubMed
- 26. Bocchino M, Rea G, Buonocore A, Lieto R, Mazzocca A, Di Domenico A, et al. Combination of acute exacerbation of idiopathic nonspecific interstitial pneumonia and pulmonary embolism after booster anti-COVID-19 vaccination. Respir Med Case Rep 2022;38:101674. ArticlePubMedPMC
- 27. Alshammari F, Abuzied Y, Korairi A, Alajlan M, Alzomia M, AlSheef M. Bullous pemphigoid after second dose of mRNA- (Pfizer-BioNTech) COVID-19 vaccine: a case report. Ann Med Surg (Lond) 2022;75:103420. ArticlePubMedPMC
- 28. Kyaw H, Shajahan S, Gulati A, Synn S, Khurana S, Nazar N, et al. COVID-19 mRNA vaccine-associated myocarditis. Cureus 2022;14:e21009.ArticlePubMedPMC
- 29. Huynh A, Kelton JG, Arnold DM, Daka M, Nazy I. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature 2021;596:565–9.ArticlePubMedPDF
- 30. Greinacher A, Selleng K, Palankar R, Wesche J, Handtke S, Wolff M, et al. Insights in ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. Blood 2021;138:2256–68.ArticlePubMedPMCPDF
- 31. Baker AT, Boyd RJ, Sarkar D, Teijeira-Crespo A, Chan CK, Bates E, et al. ChAdOx1 interacts with CAR and PF4 with implications for thrombosis with thrombocytopenia syndrome. Sci Adv 2021;7:eabl8213.ArticlePubMedPMC
- 32. Korea Disease Control and Prevention Agency (KDCA). COVID-19 vaccination adverse event status report (week 130). KDCA; 2023.
- 33. Hippisley-Cox J, Patone M, Mei XW, Saatci D, Dixon S, Khunti K, et al. Risk of thrombocytopenia and thromboembolism after COVID-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ 2021;374:n1931. ArticlePubMedPMC
- 34. Cari L, Fiore P, Naghavi Alhosseini M, Sava G, Nocentini G. Blood clots and bleeding events following BNT162b2 and ChAdOx1 nCoV-19 vaccine: an analysis of European data. J Autoimmun 2021;122:102685. ArticlePubMedPMC
- 35. Cari L, Alhosseini MN, Fiore P, Pierno S, Pacor S, Bergamo A, et al. Cardiovascular, neurological, and pulmonary events following vaccination with the BNT162b2, ChAdOx1 nCoV-19, and Ad26.COV2.S vaccines: an analysis of European data. J Autoimmun 2021;125:102742. ArticlePubMedPMC
- 36. Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, Hanson KE, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA 2021;326:1390–9.ArticlePubMedPMC
- 37. Simpson CR, Shi T, Vasileiou E, Katikireddi SV, Kerr S, Moore E, et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med 2021;27:1290–7.ArticlePubMedPMCPDF
- 38. Houghton DE, Wysokinski W, Casanegra AI, Padrnos LJ, Shah S, Wysokinska E, et al. Risk of venous thromboembolism after COVID-19 vaccination. J Thromb Haemost 2022;20:1638–44.ArticlePubMedPMCPDF
- 39. Burn E, Roel E, Pistillo A, Fernandez-Bertolin S, Aragon M, Raventos B, et al. Thrombosis and thrombocytopenia after vaccination against and infection with SARS-CoV-2 in Catalonia, Spain. Nat Commun 2022;13:7169. ArticlePubMedPMCPDF
- 40. Nicholson M, Goubran H, Chan N, Siegal D. No apparent association between mRNA COVID-19 vaccination and venous thromboembolism. Blood Rev 2022;56:100970. ArticlePubMedPMC
- 41. Pottegard A, Lund LC, Karlstad O, Dahl J, Andersen M, Hallas J, et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ 2021;373:n1114. ArticlePubMedPMC
- 42. Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, et al. Safety of the BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med 2021;385:1078–90.ArticlePubMedPMC
- 43. Health Insurance Review and Assessment Service (HIRA). September 29, "World Heart Day": heart diseases by the numbers [Internet]. HIRA; 2021 [cited 2023 Sep 1]. Available from: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020041000100&brdScnBltNo=4&brdBltNo=10428&pageIndex=1#none
- 44. Tajstra M, Jaroszewicz J, Gąsior M. Acute coronary tree thrombosis after vaccination for COVID-19. JACC Cardiovasc Interv 2021;14:e103–4.ArticlePubMedPMC
- 45. Lim Y, Kim MC, Kim KH, Jeong IS, Cho YS, Choi YD, et al. Case report: acute fulminant myocarditis and cardiogenic shock after messenger RNA coronavirus disease 2019 vaccination requiring extracorporeal cardiopulmonary resuscitation. Front Cardiovasc Med 2021;8:758996. ArticlePubMedPMC
- 46. van den Hoogen P, van den Akker F, Deddens JC, Sluijter JP. Heart failure in chronic myocarditis: a role for microRNAs? Curr Genomics 2015;16:88–94.ArticlePubMedPMC
Citations
Citations to this article as recorded by

- The Role of COVID-19 Vaccination for Patients With Atherosclerotic Cardiovascular Disease in the Upcoming Endemic Era
Kye Hun Kim
Journal of Lipid and Atherosclerosis.2024; 13(1): 21. CrossRef
 , Kye Hun Kim
, Kye Hun Kim
