ABSTRACT
- Hypertension is a significant risk factor for a variety of cardiovascular diseases, including stroke, coronary artery disease, heart failure, and peripheral arterial disease. Achieving and maintaining a specific target blood pressure (BP) is crucial for effectively reducing the risk associated with these conditions. This involves customizing treatments to meet the individual needs of patients with hypertension, ensuring that each person receives the most appropriate care for their particular circumstances. Previously, based on the findings from the ACCORD (Action to Control Cardiovascular Risk in Diabetes) study conducted over the past decade, the target BP for patients with hypertension was set at <140/90 mmHg, regardless of the patient's risk profile. However, new insights from reanalyzed data of studies such as the SPRINT (Systolic Blood Pressure Intervention Trial), the STEP (Strategy of Blood Pressure Intervention in the Elderly Hypertensive Patients) study, and ACCORD subgroup reanalysis have led to a change in this approach. These studies support a more aggressive target BP of <130/80 mmHg, especially for high-risk patients. The purpose of this article is to offer a thorough review of these updated recommendations and to explain the reasoning behind the revised target BP guidelines for individuals with hypertension.
-
Keywords: Cardiovascular risk; Blood pressure; Hypertension
INTRODUCTION
- Cardiovascular disease (CVD) remains the leading cause of mortality and morbidity worldwide [1]. Hypertension, or high blood pressure (BP), is one of the most prevalent and significant risk factors for CVD [2–4]. Fortunately, individuals can dramatically reduce their risk of developing CVD by controlling hypertension [5–9]. From a cost-effectiveness standpoint, controlling BP is more effective in reducing the risk of cardiovascular disease and mortality than any other medical intervention [10]. Years of intensive research and dedicated human effort have yielded a variety of practical strategies to combat hypertension. These include lifestyle modifications, pharmaceutical treatments, and advanced device therapies [3]. For individuals diagnosed with hypertension, establishing a target BP is crucial. This target acts as a benchmark for health professionals and patients alike, providing a measure to determine whether BP is being managed effectively [11]. The target BP varies depending on the patient's age, underlying health conditions, and overall cardiovascular risk. Recent major clinical studies have led to a reevaluation and, in many cases, a downward adjustment of the target BP, particularly for high-risk patients. This review summarizes the latest evidence for determining the target BP in the management of hypertension.
RECENT EVIDENCE FOR TARGET BP
- Numerous randomized studies have established that the threshold for systolic BP (SBP) is 140 mmHg, and for diastolic BP (DBP), it is 90 mmHg. Lowering BP to these levels has been shown to effectively reduce the risk of cardiovascular events [7–9]. In light of this evidence, there is a consensus that the target BP for patients with uncomplicated and low-to-moderate risk hypertension should be <140/90 mmHg. While some studies in the general population have indicated that a target SBP of <130 mmHg can lead to a reduction in cardiovascular events, a target of <140 mmHg is generally considered more reasonable than one of <130 mmHg [4].
- While there is limited evidence regarding the optimal DBP targets, it has been suggested that DBP values of 90 and 80 mmHg correspond to SBP values of 140 and 130 mmHg, respectively. Recently, the primary debate surrounding target BP levels has centered on whether to set the target at <140/90 or <130/80 mmHg.
- Over the past decade, there has been considerable debate regarding the optimal target BP for patients at high cardiovascular risk. The ACCORD (Action to Control Cardiovascular Risk in Diabetes) study [12] found no significant difference in the incidence of composite cardiovascular events between the intensive treatment group, which had a BP target of SBP <120 mmHg, and the conservative treatment group, with a BP target of SBP <140 mmHg. Although the ACCORD study was limited to patients with diabetes, it was a well-designed randomized trial that specifically addressed target BP. Its findings had a significant impact. Since diabetes is a high-risk factor comparable to CVD [13], the ACCORD study raised questions about the benefits of intensive BP control in high-risk patients. As a result, major guidelines around the world recommended a target BP of <140/90 mmHg, regardless of cardiovascular risk [14,15]. However, numerous observational studies and meta-analyses have suggested that an SBP of <120 mmHg may be optimal for minimizing cardiovascular risk [2,16,17]. Additionally, research has shown that patients with elevated BP (SBP, 120–129 mmHg) or prehypertension (SBP, 130–139 mmHg) are at an increased risk of developing CVD compared to those with normal BP (SBP <120 mmHg) [18].
- The ACCORD study [12] utilized a 2×2 design to examine the effects of both glucose and BP control. When analyzing patients with reasonably well-controlled glucose levels separately, it was noted that the risk of cardiovascular events was lower in the intensive treatment group that aimed for a target SBP of <120 mmHg. This evidence has led to consistent recommendations to lower the target BP in high-risk patients. However, due to the absence of supportive evidence from other well-designed randomized trials like ACCORD, there was insufficient data to alter clinical guidelines that demand high-quality evidence. This changed with the release of the SPRINT (Systolic Blood Pressure Intervention Trial) [5] results in 2015 in the United States, which reshaped the approach to target BP for hypertension in high-risk patients. The SPRINT study was a randomized trial that included high-risk patients with a substantial risk of CVD, such as those with existing CVD, older adults, individuals with chronic kidney disease (CKD), and those with a 10-year cardiovascular risk >10%. Participants were divided into two groups with target SBPs of <120 and <140 mmHg, respectively, to compare the risk of cardiovascular events based on the BP-lowering effect. The study found that the intensive treatment group, which maintained a target SBP of <120 mmHg, experienced a significant reduction in cardiovascular events compared to the standard treatment group, which had a target SBP of <140 mmHg (hazard ratio with intensive treatment, 0.75; 95% confidence interval, 0.64–0.89; P<0.001). The benefits of BP reduction were so substantial that the trial was stopped early. The SPRINT study's findings were particularly credible because the study was conducted under the auspices of the US National Institutes of Health (NIH) without pharmaceutical company involvement, which increased the academic community's confidence in the results. In 2017, the 2017 American College of Cardiology/American Heart Association (ACC/AHA) hypertension guidelines [19] revised the diagnostic criteria for hypertension from ≥140/90 to ≥130/80 mmHg, based on the SPRINT study.
- Subsequent meta-analyses [20] that included the SPRINT study and other research have consistently reported the benefits of intensive treatment for high-risk patients, leading to stronger arguments for lowering the target BP in this population. When patients who met the SPRINT study's enrollment criteria were analyzed separately in the ACCORD study, the results closely mirrored those of SPRINT [21]. Furthermore, the STEP (Strategy of Blood Pressure Intervention in the Elderly Hypertensive Patients) study [6], a randomized BP intervention trial for older patients in China released in 2021, also showed that strong BP reduction could decrease cardiovascular risk even in older patients with hypertension with multiple cardiovascular risk factors. At 1 year of follow-up in the STEP study, the mean SBP was 127.5 mmHg in the intensive treatment group and 135.3 mmHg in the standard treatment group. Based on these research findings, hypertension treatment guidelines in the United States [19], Europe [22], and Korea [11] have also adjusted the SBP target for high-risk patients from the previous <140 to <130 mmHg. It is important to note that in the SPRINT study [5], the SBP target of <120 mmHg was measured using automated office BP, a method where the patient's BP is taken three consecutive times by an automatic monitor in a quiet room without medical staff present, which reduces the white coat effect and results in lower readings than traditional in-office measurements. Given the higher frequency of side effects such as hypotension, fainting, renal function decline, and electrolyte abnormalities in the intensive BP reduction group [5,12], the target SBP for high-risk patients was set at <130 mmHg instead of <120 mmHg.
- According to the recently updated 2023 European Society of Hypertension practice guidelines [3], it is recommended that efforts be made to achieve a BP range of 120–129/70–79 mmHg in most patients up to 79 years old, provided that they tolerate the reduction in BP well. This suggests that lowering BP as much as possible may be beneficial not only for high-risk patients but also for those at low to medium risk.
TARGET BP IN SPECIFIC CONDITIONS
- Based on the hypertension treatment guidelines revised by the Korean Hypertension Society in 2022 [11], the target BPs for various diseases and clinical situations can be summarized as follows.
- Hypertension in older people
- Hypertension should be managed in older adults with appropriate consideration of their unique characteristics [23,24]. Elderly patients typically exhibit increased arterial stiffness, which leads to elevated pulse pressure and a higher incidence of isolated systolic hypertension. They also experience greater BP variability, along with a higher occurrence of masked hypertension and white coat hypertension. Furthermore, older adults often have an increased risk of side effects from antihypertensive medications due to polypharmacy for other coexisting conditions, which raises the potential for drug interactions with antihypertensive treatments. Despite these challenges, the benefits of BP reduction are evident in older adults; therefore, they should not neglect the management of high BP [5,6,25,26]. In the SHEP (Systolic Hypertension in the Elderly Program) study [26], which included 4,736 patients aged 60 years and older with isolated systolic hypertension, reducing SBP from 155 to 144 mmHg using chlorthalidone and/or atenolol led to a 32% decrease in CVD incidence. Similarly, in the HYVET (Hypertension in the Very Elderly Trial) [25] involving 3,845 patients aged 80 years and above, controlling SBP above 160 mmHg with indapamide or perindopril to achieve levels around 140 mmHg resulted in a 23% reduction in CVD incidence compared to the placebo group. For older adults with hypertension, the general target BP is <140/90 mmHg [22]. While some past studies reported no significant difference between maintaining an SBP of <140 and <150 mmHg in older adults, recent research, such as the SPRINT study [5] and STEP study [6], has shown that more aggressive BP control in older adults is even more effective in reducing CVD risk. However, older adults have a higher frequency of side effects from BP reduction than younger individuals. Therefore, particularly in frail older adults, care should be taken not to reduce BP excessively, and potential side effects should be closely monitored [3].
- Patients with high-risk profiles
- For patients with hypertension with asymptomatic organ damage or for those with three or more cardiovascular risk factors, it is recommended to control BP to <130/80 mmHg, even in the absence of complications [11]. The SPRINT study [5], which demonstrated the benefits of BP reduction in patients with high-risk factors, provides strong support for this recommendation.
- Patients with diabetes mellitus
- Hypertension is highly prevalent in patients with diabetes [27]. Controlling BP in this population is critical, as it significantly reduces the risk of CVD, including coronary heart disease and stroke, which are frequent complications of diabetes [28]. Tight BP management is also beneficial in preventing or slowing the progression of diabetic complications, such as kidney disease and retinopathy [29,30]. In a study that utilized a database from the National Health Insurance Service of Korea (NHIS) [31], approximately 240,000 patients with both hypertension and diabetes were examined. The study found that, in patients under the age of 70 years, lower BP was associated with a decreased incidence of cardiovascular events.
- However, debate continues regarding the optimal BP target for patients with diabetes [32]. Among the studies proposing target BP levels for this group, the ACCORD study [13] is particularly noteworthy. This study included 4,733 patients with diabetes who were receiving antihypertensive treatment to achieve SBP targets of either <120 or <140 mmHg. The findings revealed no significant difference in the overall incidence of cardiovascular events and mortality between the two target groups. However, the group that underwent more intensive BP-lowering therapy experienced a higher rate of side effects. As a result, for some time, a BP target of <140/90 mmHg was recommended for patients with diabetes. Several other studies, primarily sub-analyses, have similarly concluded that intensive BP-lowering therapy did not reduce the incidence of cardiovascular events in patients with diabetes [33,34]. Subsequent meta-analysis [35] have shown no difference in CVD incidence in patients with diabetes when the BP target was set below 140 mmHg compared to below 130 mmHg. Therefore, the benefits of intensive BP lowering in the general diabetic population remain unclear.
- The current Korean guideline [11] recommends a target BP of <140/90 mmHg for the general diabetic population. However, for patients with diabetes with high-risk factors, such as those with asymptomatic organ damage or at least one CVD risk factor, a more intensive BP-lowering therapy is emphasized, with a target BP set at <130/80 mmHg. This recommendation is informed by reanalyzed data from the ACCORD study [21], which demonstrated that intensive BP reduction reduced cardiovascular risk in high-risk patients, and is in line with criteria from the SPRINT study [5], as well as findings from the STEP study [6], which included 19% patients with diabetes.
- Patients with CVD
- Based on the SPRINT study [5], a target BP of <130/80 mmHg is recommended in patients with CVD (coronary artery disease, peripheral artery disease, abdominal aortic aneurysm, heart failure, or left ventricular hypertrophy).
- Patients with CKD
- Hypertension is the most prevalent comorbidity in CKD, with its prevalence estimated at 70% to 80% in stage 1 and rising to over 90% in stages 4 and 5, according to office BP measurements [36]. The primary mechanism behind high BP in CKD involves sodium retention and the activation of both the sympathetic nervous system and the renin-angiotensin system, due to a decreased glomerular filtration rate [37]. In patients with CKD, hypertension is a major risk factor for worsening renal function and the onset of CVD [38,39]. The SPRINT study [40] showed that patients with CKD who received intensive BP-lowering treatment (with a target SBP of <120 mmHg) had reduced mortality and cardiovascular risk. This finding led to the 2021 revision of the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines [41], which now recommended controlling SBP to <120 mmHg in CKD patients. However, there are limitations in directly accepting the results of the SPRINT study, because the SPRINT study excluded patients with diabetes, younger individuals (under 50 years old), and those with significant proteinuria (>1 g/day), and the BP measurement method used in the study does not align with actual clinical practices [5,42]. In the MDRD (Modification of Diet in Renal Disease) study [43] and the AASK (African American Study of Kidney Disease and Hypertension) study [44], intensive BP reduction targeting an SBP of around 130 mmHg in CKD patients did not significantly impact renal function or cardiovascular outcomes. The MDRD study enrolled 1,585 patients with an estimated glomerular filtration rate (eGFR) of 25 to 55 mL/min/1.73m2. The study maintained average BP below 107 mmHg for those under 60 years and below 113 mmHg for those over 60 years in the usual BP group. In the lower BP group, targets were below 92 mmHg for those under 60 years and below 98 mmHg for those over 60 years. Over an average follow-up of 2.2 years, there was no significant difference in the incidence of end-stage renal disease (ESRD) and death between the usual and lower BP groups [43]. However, subgroup analyses from both the randomized trial and the extended study indicated that the benefits of a lower BP target on the GFR slope and hard outcomes were primarily evident in patients with proteinuria, especially those excreting more than 1 g/day of protein [45,46]. In the AASK study, 1,094 patients with hypertension with a baseline eGFR of 20 to 65 mL/min/1.73m2 were enrolled. The usual BP group aimed for a mean BP of 102 to 107 mmHg, while the lower BP group targeted <92 mmHg. Over a follow-up of 3 to 6.4 years, the outcomes measured included a decline in eGFR by more than 50%, ESRD, and death. No significant differences were observed between the two groups [44]. However, the impact varied based on baseline proteinuria levels (P=0.02 for interaction), showing a potential benefit for patients with a protein-to-creatinine ratio of more than 0.22 g/g (about 320 mg/day), indicated by a hazard ratio of 0.73 (P=0.01) [47]. Collectively, for CKD patients with relatively high levels of albuminuria, the BP-lowering effect consistently demonstrated in several studies appears to effectively suppress the deterioration of renal function and reduce cardiovascular risk, especially compared to CKD patients with low or no albuminuria [45–47]. Based on these research findings, controlling BP to <130/80 mmHg in CKD patients with prominent albuminuria is recommended. However, it is advised for CKD patients with no or mild albuminuria or general CKD patients to maintain BP at <140/90 mmHg. For CKD patients who also have diabetes, given their higher risk profile akin to high-risk patients with diabetes, it is better to aim for a slightly lower BP of <130/80 mmHg [11].
- Patients with stroke
- Hypertension is a major risk factor for stroke recurrence and the onset of CVD in stroke patients, making BP control essential in this population [48,49]. Numerous clinical studies have demonstrated limited benefits when SBP was controlled to <130 mmHg in stroke patients [50]. In the PATS (Post-stroke Antihypertensive Treatment Study), 5,665 patients with a history of stroke or transient ischemic attack (TIA) were enrolled. Over an average follow-up period of 2 years, the group treated with 2.5 mg of indapamide achieved a BP reduction to 144/87 mmHg, which led to an approximate 30% reduction in recurrent stroke compared to the placebo group, which maintained a BP of 149/89 mmHg [51]. In the PROGRESS (Perindopril Protection Against Recurrent Stroke Study), 6,105 patients with a history of stroke or TIA were administered 4 mg of perindopril, which lowered their SBP from 147 to 138 mmHg. This treatment resulted in a 26% reduction in the risk of major cardiovascular events compared to the placebo group, where SBP remained at 147 mmHg [48]. However, a subsequent study involving 20,332 stroke patients, which used 80 mg of telmisartan to reduce SBP from 144 to 136 mmHg, did not show a decrease in the recurrence of stroke or cardiovascular events compared to the control group, which experienced a reduction from 144 mmHg to 141 mmHg (P>0.05) [52]. In the SPS3 (Secondary Prevention of Small Subcortical Strokes) study [50], 3,020 patients with symptomatic lacunar infarctions confirmed by brain magnetic resonance imaging were analyzed. The study found no difference in the recurrence of stroke or the occurrence of cardiovascular events between the intensive treatment group, which lowered SBP to 127 mmHg, and the standard treatment group, which reduced SBP to 138 mmHg. However, the authors noted a significant 63% reduction in the incidence of hemorrhagic strokes when SBP was controlled to <130 mmHg. Based on these findings, the recommended target BP for general stroke patients is <140/90 mmHg, while for those with lacunar infarction, the target is <130/80 mmHg.
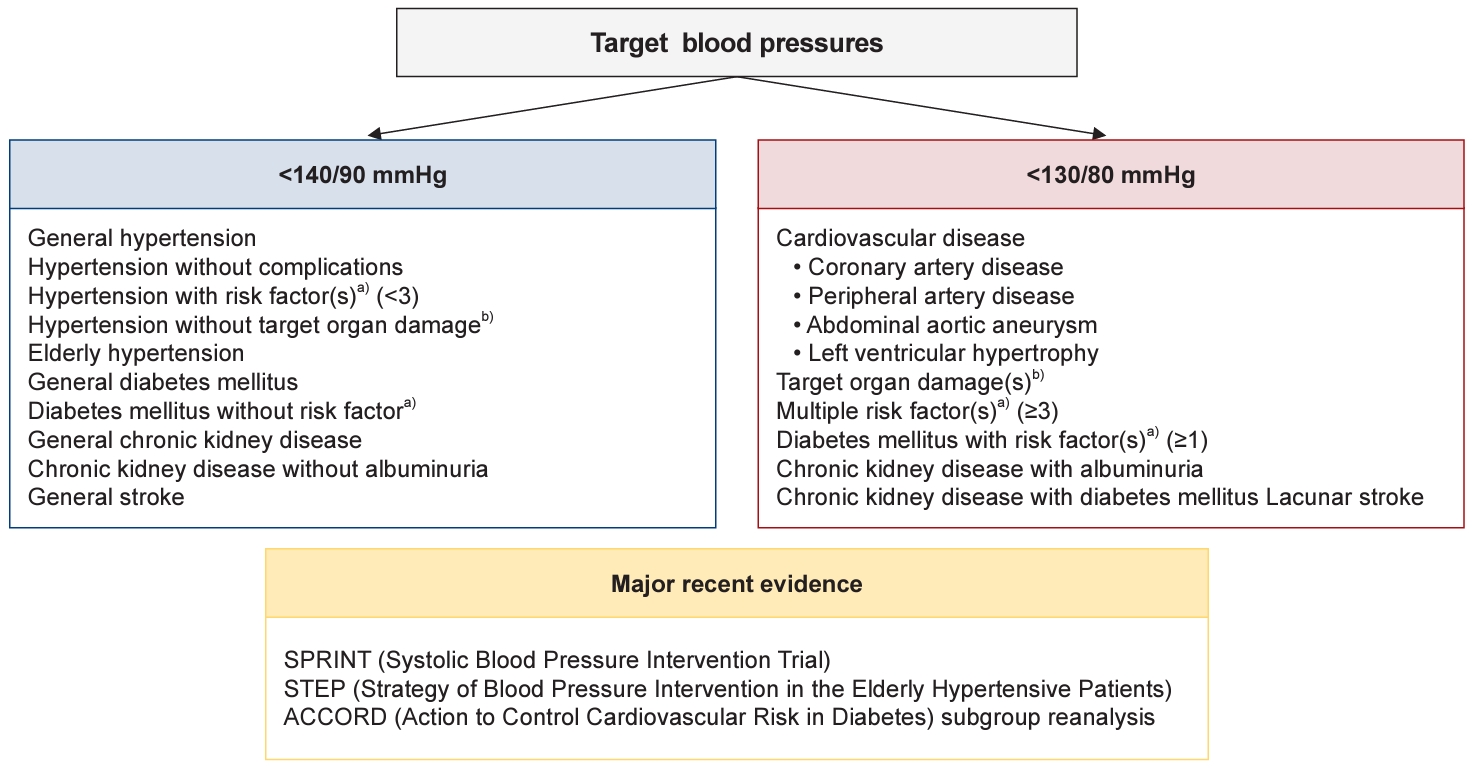
- Target BPs are summarized in Fig. 1.
LOWER LIMIT OF THE TARGET BP
- The "J curve" hypothesis posits that cardiovascular risk may increase when BP is excessively low [53,54]. This understanding of the J curve is particularly important when determining target BP levels. It is essential to recognize that in patients with CVD, an overemphasis on intensive BP-lowering therapy could result in detrimental effects due to overly reduced BP. When prescribing antihypertensive medications, it is important to consider potential side effects associated with low BP, such as syncope, deterioration of kidney function, and electrolyte imbalances [5,12]. Therefore, it is essential to consider the lower limit of the BP treatment target. Generally, it is recommended to avoid lowering treatment BP to <110/70 mmHg. Specifically, DBP is vital for maintaining coronary artery blood flow; therefore, caution is advised to prevent reducing DBP to <70 mmHg in older adults, patients with diabetes, and those with coronary artery disease or cardiac hypertrophy [55,56].
CONCLUSIONS
- In general, for patients with hypertension with a low risk of CVD, the target BP is <140/90 mmHg. However, recent findings from the SPRINT and STEP trials, as well as a reanalysis of the ACCORD trial, have highlighted the benefits of intensive BP reduction in high-risk patients. These high-risk groups include individuals with high-risk hypertension, CVD, high-risk diabetes, CKD with albuminuria, and lacunar infarction. As a result, the target BP for these patients has been set at <130/80 mmHg. For patients with diabetes, CKD, and a history of stroke, the evidence does not support a uniform target BP of <130/80 mmHg. Instead, it is recommended to consider lowering the target BP to <130/80 mmHg selectively for those with additional high-risk factors. It is crucial to recognize the appropriate target BP for each patient and to implement this knowledge in clinical practice, with the ultimate goal of improving the prognosis for individuals with hypertension.
ARTICLE INFORMATION
-
Conflicts of interest
The author has no conflicts of interest to declare.
-
Funding
The author received no financial support for this study.
Fig. 1.Target blood pressure. a)Risk factors: age (male ≥45 years, female ≥55 years), family history of early cardiovascular/cerebrovascular disease (male <55 years, female <65 years), smoking, obesity, dyslipidemia, prediabetes and diabetes mellitus. b)Target organ damage: periventricular white matter hyperintensity, microbleeds, asymptomatic stroke, left ventricular hypertrophy, albuminuria, low glomerural filtration rate, atheromatous plaque, carotid-femoral pulse wave velocity >10 m/sec, brachial-ankle pulse wave velocity >18 m/sec, coronary artery calcium score ≥400, hypertensive retinopathy (grade ≥3).
REFERENCES
- 1. Tsao CW, Aday AW, Almarzooq ZI, Anderson CA, Arora P, Avery CL, et al. Heart disease and stroke statistics: 2023 update: a report from the American Heart Association. Circulation 2023;147:e93–621.ArticlePubMed
- 2. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903–13.ArticlePubMed
- 3. Mancia G, Kreutz R, Brunstrom M, Burnier M, Grassi G, Januszewicz A, et al. 2023 ESH guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J Hypertens 2023;41:1874–2071.ArticlePubMed
- 4. Zanchetti A, Grassi G, Mancia G. When should antihypertensive drug treatment be initiated and to what levels should systolic blood pressure be lowered? A critical reappraisal. J Hypertens 2009;27:923–34.ArticlePubMed
- 5. SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103–16.ArticlePubMedPMC
- 6. Zhang W, Zhang S, Deng Y, Wu S, Ren J, Sun G, et al. Trial of intensive blood-pressure control in older patients with hypertension. N Engl J Med 2021;385:1268–79.ArticlePubMed
- 7. Brunstrom M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med 2018;178:28–36.ArticlePubMedPMC
- 8. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension. 1. Overview, meta-analyses, and meta-regression analyses of randomized trials. J Hypertens 2014;32:2285–95.ArticlePubMed
- 9. Sundström J, Arima H, Jackson R, Turnbull F, Rahimi K, Chalmers J, et al. Effects of blood pressure reduction in mild hypertension: a systematic review and meta-analysis. Ann Intern Med 2015;162:184–91.ArticlePubMed
- 10. Moran AE, Odden MC, Thanataveerat A, Tzong KY, Rasmussen PW, Guzman D, et al. Cost-effectiveness of hypertension therapy according to 2014 guidelines. N Engl J Med 2015;372:447–55.ArticlePubMedPMC
- 11. Kim HL, Lee EM, Ahn SY, Kim KI, Kim HC, Kim JH, et al. The 2022 focused update of the 2018 Korean Hypertension Society Guidelines for the management of hypertension. Clin Hypertens 2023;29:11. ArticlePubMedPMCPDF
- 12. ACCORD Study Group, Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–85.ArticlePubMedPMC
- 13. Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–34.ArticlePubMed
- 14. James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–20.ArticlePubMed
- 15. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013;31:1281–357.ArticlePubMed
- 16. Rodgers A, Ezzati M, Vander Hoorn S, Lopez AD, Lin RB, Murray CJ, et al. Distribution of major health risks: findings from the Global Burden of Disease study. PLoS Med 2004;1:e27.ArticlePubMedPMC
- 17. Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet 2014;383:1899–911.ArticlePubMedPMC
- 18. Huang Y, Su L, Cai X, Mai W, Wang S, Hu Y, et al. Association of all-cause and cardiovascular mortality with prehypertension: a meta-analysis. Am Heart J 2014;167:160–8.ArticlePubMed
- 19. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:e127–248.ArticlePubMed
- 20. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016;387:957–67.ArticlePubMed
- 21. Buckley LF, Dixon DL, Wohlford GF 4th, Wijesinghe DS, Baker WL, Van Tassell BW. Intensive versus standard blood pressure control in SPRINT-eligible participants of ACCORD-BP. Diabetes Care 2017;40:1733–8.ArticlePubMedPDF
- 22. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–104.ArticlePubMed
- 23. Lee JH, Kim KI, Cho MC. Current status and therapeutic considerations of hypertension in the elderly. Korean J Intern Med 2019;34:687–95.ArticlePubMedPMCPDF
- 24. Benetos A, Petrovic M, Strandberg T. Hypertension management in older and frail older patients. Circ Res 2019;124:1045–60.ArticlePubMed
- 25. Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887–98.ArticlePubMed
- 26. SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991;265:3255–64.ArticlePubMed
- 27. Bae JH, Han KD, Ko SH, Yang YS, Choi JH, Choi KM, et al. Diabetes fact sheet in Korea 2021. Diabetes Metab J 2022;46:417–26.ArticlePubMedPMCPDF
- 28. Patel A; ADVANCE Collaborative Group, MacMahon S, Chalmers J, Neal B, Woodward M, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007;370:829–40.ArticlePubMed
- 29. Knowler WC, Bennett PH, Ballintine EJ. Increased incidence of retinopathy in diabetics with elevated blood pressure: a six-year follow-up study in Pima Indians. N Engl J Med 1980;302:645–50.ArticlePubMed
- 30. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998;317:703–13.ArticlePubMedPMC
- 31. Kim HL, Kim HM, Kwon CH, Shin JH, Jung MH, Lee CJ, et al. Blood pressure levels and cardiovascular risk according to age in patients with diabetes mellitus: a nationwide population-based cohort study. Cardiovasc Diabetol 2020;19:181. ArticlePubMedPMCPDF
- 32. Kim HJ, Kim KI. Blood pressure target in type 2 diabetes mellitus. Diabetes Metab J 2022;46:667–74.ArticlePubMedPMCPDF
- 33. Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998;351:1755–62.ArticlePubMed
- 34. Cooper-DeHoff RM, Gong Y, Handberg EM, Bavry AA, Denardo SJ, Bakris GL, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA 2010;304:61–8.ArticlePubMedPMC
- 35. Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA 2015;313:603–15.ArticlePubMed
- 36. Sarafidis PA, Sharpe CC, Wood E, Blacklock R, Rumjon A, Al-Yassin A, et al. Prevalence, patterns of treatment, and control of hypertension in predialysis patients with chronic kidney disease. Nephron Clin Pract 2012;120:c147–55.ArticlePubMedPDF
- 37. Ku E, Lee BJ, Wei J, Weir MR. Hypertension in CKD: core curriculum 2019. Am J Kidney Dis 2019;74:120–31.ArticlePubMed
- 38. Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, et al. Blood pressure and end-stage renal disease in men. N Engl J Med 1996;334:13–8.ArticlePubMed
- 39. Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med 2005;165:923–8.ArticlePubMed
- 40. Cheung AK, Rahman M, Reboussin DM, Craven TE, Greene T, Kimmel PL, et al. Effects of intensive BP control in CKD. J Am Soc Nephrol 2017;28:2812–23.ArticlePubMedPMC
- 41. Tomson CR, Cheung AK, Mann JF, Chang TI, Cushman WC, Furth SL, et al. Management of blood pressure in patients with chronic kidney disease not receiving dialysis: synopsis of the 2021 KDIGO Clinical Practice Guideline. Ann Intern Med 2021;174:1270–81.ArticlePubMed
- 42. Dasgupta I, Zoccali C. Is the KDIGO systolic blood pressure target <120 mm Hg for chronic kidney disease appropriate in routine clinical practice? Hypertension 2022;79:4–11.ArticlePubMedPMC
- 43. Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med 1994;330:877–84.ArticlePubMed
- 44. Wright JT Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002;288:2421–31.ArticlePubMed
- 45. Sarnak MJ, Greene T, Wang X, Beck G, Kusek JW, Collins AJ, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med 2005;142:342–51.ArticlePubMed
- 46. Peterson JC, Adler S, Burkart JM, Greene T, Hebert LA, Hunsicker LG, et al. Blood pressure control, proteinuria, and the progression of renal disease: the Modification of Diet in Renal Disease Study. Ann Intern Med 1995;123:754–62.ArticlePubMed
- 47. Appel LJ, Wright JT Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 2010;363:918–29.ArticlePubMedPMC
- 48. PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358:1033–41.ArticlePubMed
- 49. Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke 2003;34:2741–8.ArticlePubMed
- 50. SPS3 Study Group, Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013;382:507–15.ArticlePubMedPMC
- 51. PATS Collaborating Group. Post-stroke antihypertensive treatment study: a preliminary result. Chin Med J (Engl) 1995;108:710–7.PubMed
- 52. Yusuf S, Diener HC, Sacco RL, Cotton D, Ounpuu S, Lawton WA, et al. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 2008;359:1225–37.ArticlePubMedPMC
- 53. Foy AJ, Filippone EJ, Schaefer E, Nudy M, Ruzieh M, Dyer AM, et al. Association between baseline diastolic blood pressure and the efficacy of intensive vs standard blood pressure-lowering therapy. JAMA Netw Open 2021;4:e2128980.ArticlePubMedPMC
- 54. Kimm H, Mok Y, Lee SJ, Lee S, Back JH, Jee SH. The J-curve between diastolic blood pressure and risk of all-cause and cardiovascular death. Korean Circ J 2018;48:36–47.ArticlePubMedPMCPDF
- 55. Bohm M, Schumacher H, Teo KK, Lonn EM, Mahfoud F, Mann JF, et al. Achieved blood pressure and cardiovascular outcomes in high-risk patients: results from ONTARGET and TRANSCEND trials. Lancet 2017;389:2226–37.ArticlePubMed
- 56. Messerli FH, Kupfer S, Pepine CJ. J curve in hypertension and coronary artery disease. Am J Cardiol 2005;95:160. ArticlePubMed
Citations
Citations to this article as recorded by



